Abstract
Prosopis laevigata (mesquite) plants can tolerate drought, thermal stress, alkalinity, salinity, and toxicity of heavy metals, making them attractive for phytoremediation. Nonetheless, most of these features have been studied under controlled laboratory conditions. In this work, the bioaccumulation of heavy metals in a free-living population of P. laevigata trees growing in a heavily metal-contaminated site (aluminum, chromium, iron, titanium, copper, and zinc) was analyzed. Furthermore, crystal phases of mineral nutrients and trace elements found in P. laevigata tissues were determined by X-ray diffraction. P. laevigata trees accumulated 705 (± 17), 47,064 (± 1459), 14,800 (± 401) and 30,000 (± 1719) mg/kg of Cu, Zn, Fe and Al, confirming the potential of these plants to hyper-accumulate metals. The X-ray diffraction analysis showed that P. laevigata trees can chelate Al3+ with phosphates to form orthorhombic crystals of aluminum phosphate (AlPO4) in the tissues. This aluminum chelation was probably a mechanism of tolerance used by the plant. The inoculation of seedlings with the endophytic Bacillus cereus MH778713 did not prevent Cr-accumulation in the plant but increased metal tolerance and seedling development. These results highlight the use of P. laevigata and B. cereus MH778713 together as tools for heavy metal bioremediation, particularly on arid and semiarid soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contamination of soils, air and water by heavy metals derived from anthropogenic activities, like mining, farming and industrial processes, is an increasing problem that threatens all living organisms. Heavy metal pollution problems arise when the heavy metals are mobilized into the soil and then absorbed by the plants or transported to the groundwater (Wuana and Okieimen 2011). The interaction of heavy metals with cations like calcium, magnesium, and potassium present in soils determines the grade of toxicity of these metals (Palansooriya et al. 2020). The content of organic matter in soils also plays an important role in blocking the toxic action of heavy metals (Stefanowicz et al. 2020). The increasing concentration of toxic elements, in the environment, produces physiological and genetic changes in the fauna and flora of the ecosystem and, in the long-term, human health risks (Singh et al. 2016).
The term phytoremediation refers to a technology based on the use of plants, naturally or genetically modified, or microorganism-plant associations, for the remediation of polluted environments. Phytoremediation is clean, simple, cost-effective and not environmentally disruptive (Tiodar et al. 2021). On the other hand, bioremediation can be used to remove pollutants such as synthetic organic compounds, xenobiotics, heavy metals and organic hydrocarbons from soils and water (Jaiswal and Shukla 2020).
Semiarid regions are described with an aridity index (ratio of total annual precipitation to potential evapotranspiration) between 0.20 and 0.50, soils of these regions are characterized by a poor structure, low content of organic matter and reduced water retention (Garcia-Franco et al. 2018). Prosopis genus contains more than 45 species of spiny trees and shrubs widely distributed in arid and semiarid regions of America, Africa, and Asia, able to produce large amounts of biomass (14.5 T ha−1 yr−1) under water deficit conditions (Felker et al. 1983). Plants belonging to the genus Prosopis can tolerate alkalinity, high temperatures, salinity, and drought stress, besides fixing nitrogen (associated with nitrogen-fixing bacteria) and adding fertility to soils.
Experiments of Prosopis plants under laboratory conditions have shown tolerance and hyper-accumulation of lead (Aldrich et al. 2004; Buendia-Gonzalez et al. 2019), chromium (Aldrich et al. 2003), arsenic (Aldrich et al. 2007; Mokgalaka-Matlala et al. 2008), copper (Zappala et al. 2014) and zinc (Khan et al. 2015). The mechanisms by which Prosopis plants can do this remain unclear. Aldrich et al. (2003, 2007) reported that Prosopis can actively uptake and reduce Cr (VI) to Cr (III) or As (V) to As (III). Endophyte Bacillus of Prosopis laevigata isolated from root nodules improved plant tolerance and seed dormancy breakage under chromium-stress experiments (Ramírez et al. 2019, 2020). However, little is known about the capability of native P. laevigata trees to accumulate and tolerate heavy metals under wildlife conditions.
The mineral nutrient composition of a living organism is defined as ionome (Salt et al. 2008). Mineral uptake in plants is regulated by both genetic and environmental factors, thus plants can control ionomic homeostasis by regulating the uptake, transport and accumulation (Zhang et al. 2021). The development of high-throughput chemistry analysis has made it possible to evaluate the plant ionome in response to different environmental conditions or plant species (Watanabe et al. 2016, 2021).
Wavelength-dispersive X-ray fluorescence (WDXRF) spectroscopy is a powerful tool used for elemental analysis applications. This technique allows directly analyzing solid samples with multi-element identification, performing qualitative and quantitative determinations. It is highly sensitive to heavy elements, with good resolution for light chemical elements (Marguí et al. 2007). X-ray diffraction (XRD), on the other hand, is a technique used for characterizing crystalline materials; it provides information on structures, phases, crystal orientations, grain size, deformation, and crystal defects (Bunaciu et al. 2015). Therefore, both chemistry assessments can be useful for plant ionome analysis.
This work aimed to investigate the elemental composition of P. laevigata plants growing under free-living and laboratory conditions, both subjected to heavy metal stress, using high-throughput elemental analysis technologies (WDXRF spectrometry and X-ray diffraction). Ionomic data showed changes in the metal content of P. laevigata tissues in adaptation to heavy metal stress, confirming the potential of mesquite plants for the phytoremediation of metal-contaminated areas.
Materials and methods
Site and sample collection
The site of the study was a free-living population of Prosopis laevigata trees in the deserted region of Chietla, Puebla, Mexico (18°31′27. 5″ N, 98°35′02.0″ W), which is a heavy metal-contaminated region due to mining activities. The region of Chietla is located at 1079 m above sea level. Tissues of twenty P. laevigata trees were collected, oven-dried at 60 °C for 72 h, ground in a mill, pooled together and divided into two composed samples for further analysis. Leaf samples were taken from the main branches at 2 m height; stem samples were taken at 1.2 m from the soil surface; root samples were taken at 50 cm depth. For each P. laevigata tree, a rhizospheric and superficial soil sample was taken at 20 cm deep and surface, for pH analysis. Furthermore, pods from adult trees were taken to the laboratory to obtain seeds for seedlings experiments under hydroponic conditions.
Soil analysis and determination of pH
Each tree’s rhizospheric and superficial soil samples were sieved and pooled together for metal soil composition and pH analysis. Thus, two composite rhizospheric soil samples or superficial soil samples were measured three times to get three pH values for each type of soil. One gram of composite soil sample, previously dried at 50 °C for 48 h, was weighed and transferred into a 100 mL beaker containing 10 mL of deionized water. The suspension was stirred well with a glass rod and allowed to stand for 24 h. The supernatant was used to measure pH in a potentiometer.
Two composite rhizospheric soil samples were used for metal composition analysis using wavelength-dispersive X-ray fluorescence (WDXRF) spectrometry, XRD (X-ray diffraction), and atomic absorption spectrometry.
WDXRF (wavelength-dispersive X-ray fluorescence) spectrometry
Soil metal composition
10 g of each composite rhizospheric soil sample was subjected to 500 °C for 48 h; then, samples were finely powdered and then press into a pellet using wax binders for WDXRF analysis (Ramírez et al. 2019). The quantitative analysis of the multi-elements was carried out using a Bruker S8-Tiger WDXRF analyzer equipped with a Rhodium (Rh) tube as an X-ray source, different collimators and filters, a flow proportional counter for light elements (Na to V), a scintillation counter for heavy elements (Cr to Pb), and a high-pressure goniometer for theta and 2 theta angles. Analyses were conducted according to ISO9516-1: 2003 using the QUANT-EXPRESS method (fundamental parameters) which includes a unique multipurpose calibration method using certified standards (STG2). The total analysis time for each sample was 20 min with a sensitivity of 20 mg/kg for most elements. Each composite sample was analyzed three times in the WDXRF equipment.
Tissues from free-living P. laevigata trees
Two composite samples of leaf, stem, and root were calcinated at 500 °C for 44 h. Ashes were sieved and combined with wax as a binder to prepare pellets to be used in WDXRF analysis (Ramírez et al. 2019). Each composite sample was analyzed three times in the WDXRF analyzer.
Tissues from P. laevigata seedlings
Composite samples from twelve inoculated and uninoculated seedlings of P. laevigata growing over six months with chromium (25 mg/L), iron (2000 mg/L), zinc (3000 mg/L), or without metal supplementation in Jensen’s medium were analyzed by WDXRF spectrometry. Composite samples were calcinated, and ashes were finely powdered and then press into a pellet for WDXRF analysis as described before. Each composite sample was analyzed three times in the WDXRF equipment.
Bioaccumulation factor
Metal concentrations measured by WDXRF were used to calculate the bioaccumulation factor (BF).
XRD (X-ray diffraction) assay
The WDXRF is a non-destructive technique used to measure elemental composition. Therefore, the same composite samples used for WDXRF analysis were reused for X-ray diffraction analysis to identify crystalline compounds in plant tissues and rhizospheric soils. The X-ray diffraction (XRD) assay was performed using a Bruker D8 Discover X-ray diffractometer operated at 40 kV and 40 mA applying the CuKα1 radiation (1.5406 Å). The detector of scintillation is at the angle 2-theta close to zero (~ 4°), in a system connected to a radiation recording measurement as a function of the angle 2 thetas known as λ, the estimated resolution of the interplanar distances was greater than 0.02 Å. The XRD pattern of the sample was identified with the Powder Diffraction File PDF-4 + 2018 from the International Centre of Diffraction Data (ICDD). The input data for the Rietveld refinement (space group, cell parameters, and atomic positions) were taken from the database format PDF-4 + 2018. To model the background a polynomial approach was used, and a pseudo-Voigt function was selected for the profile form. After this, the shape parameters, zero shift, scale factor, unit cell parameters, U, W, and V profile coefficients were refined.
Atomic absorption spectrometry
Two composite soil and tissue samples were oven-dried at 60 °C for 72 h, finely ground, sieved, weighed, and digested with nitric acid 65% (Suprapur, Merck, Germany). To do it, 0.5 g of soil or tissue composite sample was weighed into a microwave vial. Afterward, 10 ml nitric acid (65%) was added to the vial. Digestion was performed at 175 °C for 5 min in a microwave oven (CEM-MarsX, CEM corporation, Mathews, NC). Digested samples were brought to 60 ml by adding deionized water, then filtered with a Whatman paper (GE Healthcare, UK) for metal composition analysis. Cu, Zn, Fe, Cr, Ca, and K concentrations were measured by an atomic absorption spectrometer (Agilent, Spectra 55B, Australia). Three technical replicates for each composite sample were performed.
Seedlings experiments: inoculated and uninoculated P. laevigata subjected to metal stress
Seeds of P. laevigata taken from the site of the study were scarified with concentrated sulfuric acid (98%) for 20 min in constant agitation and then rinsed several times with deionized sterile water to remove the sulfuric acid excess. Scarified seeds were then placed on agar (1%) for 3 days in the dark for germination and transferred to bottles with Jensen’s medium supplemented with 25 mg/L of chromium (VI); 2000 mg/L of iron, or 3000 mg/L of zinc. Controls were not supplemented with any metal. The composition of Jensen’s medium was as follows (g/L): KNO3 0.5, K2HPO4 0.2, MgSO4 0.2, NaCl 0.2, CaHPO4 1.0, FeCl3 0.04. Jensen’s medium was supplemented with 1 ml/L of trace metal solution, whose composition in g/L was: H3BO3 2.26, MnSO4*4H2O 2.03, ZnSO4*7H2O 0.22, CuSO4*5H2O 0.08, Na2Mo4*2H2O 0.09. Twelve plants for each treatment were incubated in a climate chamber at 20 °C with 16 h of light alternating with 8 h of darkness (Ramírez et al. 2019). For the inoculated seedlings assay, the procedure was similar but before transferring P. laevigata seedlings into the hydroponic conditions, Bacillus cereus MH778713 was inoculated into Jensen’s medium at a final concentration of 1 × 107 CFU/ml. To do it, B. cereus MH778713 was grown overnight in YM liquid medium (yeast extract 1.0 g, mannitol 10 g, dipotassium phosphate 0.5 g, sodium chloride 0.1 g, glutamic acid 0.01 g per liter, pH 7.0), then centrifuged, washed and resuspended in sterile deionized water. The experiment was run for six months when true leaves emerged. Plant growth (fresh and dry weight) was evaluated for each treatment. After taking the measurements, seedlings were dried, cut into 1 cm fragments, crushed, and calcinated for WDXRF and XRD analysis.
Statistical analysis
The data obtained from the dry and fresh weight of plants exposed to Zn, Fe and Cr were subjected to statistical analysis using one-way analysis of variance (ANOVA), and significant differences between mean values were determined by the Tukey–Kramer Post Hoc tests or least significant difference using Microsoft 365 Excel for Windows. A significance level of p < 0.05 was used to determine the significant differences in data.
Results and discussion
The composite rhizospheric samples had 3.38% (± 0.33) of organic matter, 2.01 (± 0.10) dS/cm of soil conductivity, 1.52 (± 0.03) g/ml of apparent density, and 11.81% (± 0.05) of available nitrogen. Texture analysis of rhizospheric soil resulted in 56% (± 0.075) sand, 24% (± 0.078) loam, and 25% (± 0.09) clay. The wavelength-dispersive X-ray fluorescence (WDXRF) spectrometry was used to determine the abundance of chemical elements in rhizospheric soil and plant organs of a P. laevigata population growing in a metal-contaminated zone. The results showed the ability of P. laevigata trees to bioaccumulate potassium (K), calcium (Ca), phosphate (P), magnesium (Mg), silicon (Si), sulfur (S), iron (Fe), strontium (Sr), sodium (Na), aluminum (Al), zinc (Zn), and copper (Cu) (Table 1).
Each value is the mean of two composed samples, and the error is presented in parentheses. N. D. means not detected
P. laevigata free-living trees of this study were naturally established in a hostile soil containing 53,100 (± 446) mg Al/kg, 47,450 (± 199) mg Fe/kg, 402 (± 96) mg Cu/kg, 322 (± 46) mg Cr/kg, 5100 (± 128) mg Ti/kg and 142 (± 10) mg Zn/kg. High concentrations of metals like aluminum (30,000 mg/kg), iron (14,800 mg/kg), zinc (47,064 mg/kg), and copper (705 mg/kg) were found in tissues of P. laevigata; showing the potential of these plants to absorb toxic elements (Table 1). Bioaccumulation factors for Zn, S, P, K and Ca were 113, 20, 18, 9 and 7, determined from measurements in Table 1. However, chromium and titanium were undetected in tissue plants despite their high concentration in soil (Table 1). Knowing that the detection limit of our WDXRF assay was 20 mg/kg, further validation of WDXRF spectrometry data was performed using atomic absorption spectrometry (AAS). The results of tissue accumulation of copper, zinc, iron, chromium, calcium, and potassium determined by AAS are shown in Table 2. The detection limit of AAS was 0.04 mg/kg and provided different element concentrations compared to WDXRF (Tables 1 and 2). There was no linear relationship between values obtained with AAS and WDXRF for Cu and Zn and the R2 values were lower than 0.39. Conversely, a positive linear relationship between AAS and WDXRF was observed for potassium (y = 11.43x + 17,036), calcium (y = 35.15x – 161,678) and iron (y = 39.17x + 950) with R2 values bigger than 0.93.
Since mobilization and, hence, the bioavailability of metals in soil depends on the crystalline phases, an X-ray diffraction (XRD) analysis of P. laevigata tissues and their rhizospheric soil was performed (Fig. 1 and 2).
The main crystalline structures found in soils were anorthite sodium (Al1.77Ca0.64Na0.32O8Si2.28), anorthoclase (AlK0.14Na0.85O8Si3), enstatite/ferrosilite (Mg1.56Fe0.44Si2O6) and moganite (SiO2). We presume that the elements of these mineralogical phases were scarcely available to the plants because metals bound to silicate structures are scarcely bioavailable. Moreover, when soil pH is above 7, metals tend to form insoluble metal mineral phosphates and carbonates limiting their bioavailability. In fact, rhizospheric and surface soil pH were measured, being 7.2 ± 0.9 and 8.3 ± 0.8, respectively. The diffractogram obtained from the XRD analysis of root samples (Fig. 1) showed peaks relating to three crystalline phases, calcite (CaCO3) in the rhombohedral phase, sylvite (KCl) in cubic phase, and aluminum phosphate (AlPO4) in the orthorhombic phase. The same three elements were found in stem samples, almost in the same proportions (Fig. 2). When leaf samples were analyzed by XRD (Fig. 2), two compounds, CaCO3 (in rhombohedral phase) and KCl (in cubic phase), were in common with those compounds found in the root and stem samples. Aluminum phosphate (AlPO4), namely berlinite, was also identified, but in the hexagonal phase. Two compounds, absent in the root or stem, were identified in leaves, fairchildite (K2Ca(CO3)2) in the hexagonal phase and aluminum iron phosphate (Al0.67Fe0.33PO4) in the orthorhombic phase.
P. laevigata has been reported as a chromium-hyperaccumulator-plant. These Cr-bioaccumulation traits have been described in seedlings growing in agar-based media or hydroponic conditions; however, in our free-living P. laevigata trees, chromium was not detected by WDXRF spectrometry. To clarify this contrasting result, the ability of P. laevigata seedlings to bioaccumulate chromium, iron, and zinc, under hydroponic conditions, was assessed by WDXRF spectrometry (Fig. S1). The WDXRF diffractograms of plants exposed to Cr, Fe, or Zn clearly showed the KA and KB radiation emission for the corresponding metal, but not the sample of control plants (Fig. S1 D). An elevated concentration of essential elements like K, P, Ca and S were detected in all conditions (Fig. S1).
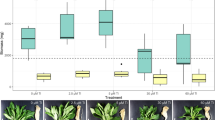
P. laevigata seedlings were able to bioaccumulate up to 3100 (± 618) mg Cr/kg, 9400 (± 1230) mg Zn/kg, and 4000 (± 1398) mg Fe/kg (Fig. 3A), representing a bioaccumulation factor of 123, 3, and 2, calculated as the ratio of element concentration in plant and element concentration in the medium knowing that culture medium contained 25.2 mg Cr/kg, 3017.5 mg Zn/kg and 2019.4 mg Fe/kg. The presence of chromium, iron, or zinc in Jensen’s medium increased the plant content of phosphorous (P) by 6- to eightfold (Fig. 3A). Our results showed that P. laevigata could uptake Cr from culture medium under hydroponic conditions but no from rhizospheric soil, even when Cr concentration of rhizospheric soil was eightfold higher than hydroponic conditions.
Ionomic analysis of P. laevigata seedling determined by wavelength-dispersive X-ray fluorescence (WDXRF) spectrometry. A Uninoculated seedlings growing for six months in Jensen’s medium supplemented with 3000 mg/L of Zn, 25 mg/L of Cr, and 2000 mg/L of Fe. B Seedling inoculated with B. cereus MH778713 growing for six months in Jensen’s medium supplemented with 3000 mg/L of Zn, 25 mg/L of Cr, and 2000 mg/L of Fe
The pH of rhizospheric soil (7.2 ± 0.9) could not be the factor preventing Cr uptake on wild P. laevigata trees because the pH of the hydroponic medium containing 25 mg Cr/L (pH = 7.28 ± 0.15) was similar. However, physical and chemical characteristics of rhizospheric soil (ionic force, organic matter, clay minerals, cation exchange capacity) can be the factors determining the solubility and therefore availability of heavy metals to the plants.
The root nodules of wild P. laevigata trees were colonized by Bacillus species with important chromium-accumulation characteristics (Ramírez et al. 2019). To rule out that the Cr-hyperaccumulator MH778713 strain, isolated from P. laevigata nodules, could be impairing the Cr uptake of plants, an absorption assay inoculating seedlings of P. laevigata with Bacillus cereus MH778713 was performed (Fig. 3B). P. laevigata seedlings inoculated with B. cereus MH778713 bioaccumulated up to 30,334 (± 4618) mg Cr/kg, representing a bioaccumulation factor of 1213. Cr-accumulation in inoculated seedlings was tenfold higher than in uninoculated P. laevigata seedlings (Fig. 3A, B). Inoculated P. laevigata seedlings accumulated 2.4-fold more iron and 1.7-fold less zinc than uninoculated seedlings (Fig. 3A, B). In all conditions, the Ca-accumulation of inoculated P. laevigata seedlings was 3.3-fold to tenfold higher than uninoculated seedlings. In contrast to inoculated seedlings, the presence of chromium and zinc increased the content of phosphorous and sulfur in uninoculated seedlings (Fig. 3A), probably to cope with metal stress. Increased P and S assimilation can be required for plants to synthesize thio-rich compounds, such as glutathione, phytochelatins, and metallothioneins, which play important roles in metal immobilization, sequestration, and detoxification (Li et al. 2023).
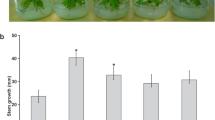
Furthermore, the effect of chromium, iron, and zinc stress on plant development was analyzed in inoculated and uninoculated P. laevigata seedlings (Fig. 4).
Effect of chromium, iron, and zinc in P. laevigata seedlings development. Blue bars represent uninoculated seedlings growing in Jensen’s medium for six months. Orange bars represent seedlings inoculated with B. cereus MH778713 growing in Jensen’s medium for six months. Different letters indicate significant differences between treatments according to the least significant difference at p = 0.05. *means significant difference p < 0.05
Compared with their controls, uninoculated and inoculated seedlings treated with chromium (25 mg/L) or zinc (3000 mg/L) displayed a significant reduction of dry and fresh weight (Fig. 4). At any condition evaluated, inoculated seedlings had higher plant weight compared to uninoculated seedlings (Fig. 4).
Discussion
The ionomic profile of wild P. laevigata trees growing in a heavy metal-contaminated site displayed the bioaccumulation of toxic elements. The accumulation of potentially toxic metals in plants depends on various factors including plant species, organs, mineral concentration and mineralogical phases present in the environment. Thus, depending on Prosopis species and the heavy metal concentration in soil, free-living trees of P. laevigata have been shown to accumulate up to 14, 19, 24, 104, 427, and 1331 mg/kg of Pb, Cd, Cu, Fe, Cr, and Zn (Beramendi-Orosco et al. 2013; Heredia et al. 2022; Khan et al. 2015; Muro-González et al. 2020; Senthilkumar et al. 2005). Our results showed that P. laevigata trees accumulated 705, 47,064, 14,800 and 30,000 mg/kg dry weight of Cu, Zn, Fe and Al, confirming the potential of these plants to hyper-accumulate metals. Aluminum is one of the most abundant metals in soils, which is normally bounded to silicates (e.g., anorthoclase, anorthite) and other nonphytotoxic mineral complexes. Under acid soil conditions or acidification by the rhizospheric microorganism, aluminosilicates can release toxic Al3+ cations into the soil, causing toxicity to the plants (Bojorquez-Quintal et al. 2017). Plants have developed two main mechanisms of tolerance, Al3+ exclusion from root cells and internal Al3+ ion tolerance (Brunner and Sperisen 2013). Our results suggest that P. laevigata trees used an internal tolerance mechanism by chelating Al3+ cations with phosphates to form aluminum phosphate (AlPO4) in the orthorhombic phase in both roots and stem (Fig. 1 and 2). Nonetheless, leaves accumulated aluminum phosphate (AlPO4) in the hexagonal phase. The crystalline phase change of AlPO4 could be due to the different mineral composition of each plant tissue; leaves, for example, contained 47% of calcite (CaCO3) in rhombohedral form while roots and stems contained 92% and 97% of CaCO3 in the same mineralogical phase. The high content of calcium carbonate (calcite) found in roots and stems could be helping plants to cope with heavy metal toxicity, because calcite increases pH, enhancing the precipitation of metal carbonates (Ali et al. 2020). Al3+ toxicity can be the most deleterious limiting factor in acid soils (pH < 5.0), since P. laevigata trees accumulated 30,000 mg/kg of Al, it will be interesting to test the potential of P. laevigata for Al-phytoextraction in acid soils.
Conversely, P. laevigata trees did not accumulate chromium (Table 1) or scarcely (Table 2), albeit the Cr concentration of rhizospheric soil was 322 (± 46) mg/kg (Table 1). When P. juliflora trees were grown in a tannery effluent contaminated soil containing 2542 mg Cr/kg, trees were able to accumulate up to 427 mg Cr/kg in roots (Khan et al. 2015). Under laboratory conditions, where culture medium or substrate is supplied with K2CrO4, seedlings of P. laevigata or P. juliflora can accumulate up to 20 to 10,983 mg Cr/kg (Aldrich et al. 2003; Buendia-Gonzalez et al. 2012, 2019; Rai et al. 2004). In line with these studies, our P. laevigata seedlings subjected to 25 mg Cr/L in hydroponic systems accumulated up to 3100 mg Cr/kg of plant ashes (Fig. 3A). The factors inhibiting Cr uptake in our P. laevigata trees are not clear, but probably the high content of calcium-aluminum–silicate mineral forms in soils (Fig. 1 and 2), the low Cr concentration in soil (322 mg/kg) and the high soil pH were the culprits of blocking Cr uptake. Cation exchange in soil and hydroponic conditions is completely different. The hydrous aluminosilicates (clay minerals) of soils have strong physical and chemical interactions with dissolved species, especially with heavy metals (Uddin 2017). Thus, the high adsorbing capacity of clay minerals of rhizospheric soils can be one factor blocking Cr uptake in wild P. laevigata trees. Under hydroponic cultures (Fig. 3), conversely, chromium amorphous structures were freely available to plants for bioaccumulation (Grigatti et al. 2015).
When P. laevigata seedlings were inoculated with B. cereus MH778713 and grown under hydroponic conditions without any metal stress, a plant growth promotion was observed (Fig. 4). This was likely the result of higher P, K, Ca, and Fe uptake (Fig. 3B), since inoculated seedlings accumulated 3.5-, 2.1-, 3.5- and 9.3-fold more minerals than the uninoculated seedlings. Zn- or Cr-stress induced higher phosphorus and sulfur assimilation in plants (Fig. 3). Under heavy metal stress, thio-rich compounds, such as glutathione, phytochelatins, and metallothioneins, are induced to keep the metal ion homeostasis by binding with heavy metal ions (Li et al. 2023). In fact, there is a differential metallothionein induction in response to cadmium, copper or zinc suggesting that each metallothionein has a specific role in P. juliflora (Usha et al. 2009).
Under all hydroponic conditions tested, chromium was the most toxic metal (Fig. 4). High concentrations of hexavalent chromium disrupt plant tissues integrity (Scoccianti et al. 2008; Sharma et al. 2003), disturbing the biosynthesis of chlorophyll (Shanker et al. 2005) and germination process (Ramírez et al. 2020). Metabolic alterations caused by hexavalent chromium promote the expression of enzymes and metabolites responsible for the detoxification and elimination of ROS (reactive oxygen species) (Shanker et al. 2005). The phytotoxic effects of Cr in uninoculated seedlings were associated with a reduction of K and Ca uptake (Fig. 3A) affecting probably potassium physiological processes that influence plant growth and metabolism, calcium sensors, plant cell walls and membranes. Potassium plays a crucial role in photosynthetic activity, osmoregulation, stomatal movements, energy transfer, phloem transport, and cation–anion balance. It also plays a mitigating role in various abiotic stresses such as drought, salinity, and metal toxicity (Wang et al. 2013). Phytotoxic effects of Cr in P. laevigata seedlings were also associated with an eightfold increase in phosphorus accumulation (Fig. 3A), and P reduces the heavy metal toxicity diluting the metal or forming a metal complex avoiding the transit of the toxic metal (Sarwar et al. 2010).
The presence of zinc in the environment also affects mesquite plant development (Fig. 4). High levels of zinc cause morphological, biochemical, and physiological disorders (Balafrej et al. 2020), starting with the disruption of the nuclear membrane and the loss of the integrity of cell organelles (Dong et al. 2006). Wavelength-dispersive X-ray fluorescence analysis (Table 1, Fig. 3A) showed the accumulation of zinc in mesquite plants up to 47,064 mg/kg under free-living conditions or 9400 mg/Kg under hydroponic conditions. Zinc hyperaccumulating plants, unlike chromium, can accumulate high concentrations of zinc in aerial tissues (Reeves and Baker 2000).
There was some variation in the elemental content of samples determined by atomic absorption spectroscopy and WDXRF (Tables 1 and 2), with WDXRF’s tendency to show higher element concentrations. The differences can be attributed to the physical interaction between the elements during the analysis. In the dry chemical analysis (WDXRF), all elements of the samples are in strong contact with each other, generating interferences of the fluorescence emission between elements, which is called the matrix effect. Wet chemistry analysis (atomic absorption spectroscopy), on the other hand, requires sample digestion with acid, reducing or eliminating element interactions.
Conclusion
P. laevigata plants growing under free-living and/or laboratory-controlled conditions can accumulate high concentrations of Zn, Fe, Al, and Cr, confirming the potential of this plant to hyper-accumulate heavy metals. The X-ray diffraction analysis showed that P. laevigata trees can chelate Al3+ cations with phosphates to form orthorhombic phases of aluminum phosphate (AlPO4), probably as an internal aluminum tolerance mechanism. The inoculation of seedlings with an endophytic Bacillus did not prevent Cr-accumulation in the plant but increased metal tolerance and seedling development. Our results highlight the use of P. laevigata with Bacillus cereus MH778713 as valuable tools for heavy metal bioremediation of arid lands.
References
Aldrich MV, Gardea-Torresdey JL, Peralta-Videa JR, Parsons JG (2003) Uptake and reduction of Cr (VI) to Cr (III) by mesquite (Prosopis spp.): chromate–plant interaction in hydroponics and solid media studied using XAS. Environ Sci Technol 37(9):1859–1864. https://doi.org/10.1021/es0208916
Aldrich MV, Ellzey lJ, Peralta-Videa JR, Gonzalez JH, Gardea-Torresdey JL (2004) Lead uptake and the effects of EDTA on lead-tissue concentrations in the desert species mesquite (Prosopis spp.). Int J Phytoremed 6(3):195–207. https://doi.org/10.1080/16226510490496357
Aldrich MV, Peralta-Videa JR, Parsons JG, Gardea-Torresdey JL (2007) Examination of arsenic (III) and (V) uptake by the desert plant species mesquite (Prosopis spp.) using X-ray absorption spectroscopy. Sci Total Environ 379(2–3):249–255. https://doi.org/10.1016/j.scitotenv.2006.08.053
Ali U, Shaaban M, Bashir S, Fu Q, Zhu J, Shoffikul Islam M, Hu H (2020) Effect of rice straw, biochar and calcite on maize plant and Ni bio-availability in acidic Ni contaminated soil. J Environ Manage 259:109674. https://doi.org/10.1016/j.jenvman.2019.109674
Balafrej H, Bogusz D, Triqui ZEA, Guedira A, Bendaou N, Smouni A, Fahr M (2020) Zinc hyperaccumulation in plants: a review. Plants 9(5):562. https://doi.org/10.3390/plants9050562
Beramendi-Orosco LE, Rodriguez-Estrada ML, Morton-Bermea O, Romero FM, Gonzalez-Hernandez G, Hernandez-Alvarez E (2013) Correlations between metals in tree-rings of Prosopis julifora as indicators of sources of heavy metal contamination. Appl Geochemistry 39:78–84. https://doi.org/10.1016/j.apgeochem.2013.10.003
Bojorquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017) Aluminum, a friend or foe of higher plants in acid soils. Front Plant Sci 8:1767. https://doi.org/10.3389/fpls.2017.01767
Brunner I, Sperisen C (2013) Aluminum exclusion and aluminum tolerance in woody plants. Front Plant Sci 4:172. https://doi.org/10.3389/fpls.2013.00172
Buendia-Gonzalez L, Estrada-Zuñiga ME, Orozco-Villafuerte J, Cruz-Sosa F, Vernon-Carter EJ (2012) Somatic embryogenesis of the heavy metal accumulator Prosopis laevigata. Plant Cell Tiss Organ Cult 108:287–296. https://doi.org/10.1007/s11240-011-0042-4
Buendía-González L, Cruz-Sosa F, Rodríguez-Huezo M, Barrera-Díaz C, Hernández-Jaimes C, Orozco-Villafuerte J (2019) In Vitro simultaneous accumulation of multiple heavy metals by Prosopis laevigata seedlings cultures. Rev Mex Ing Quím. 18:1167–1177. https://doi.org/10.24275/uam/izt/dcbi/revmexingquim/2019v18n3/Buendia
Bunaciu AA, Udriştioiu EG, Aboul-Enein HY (2015) X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem 45(4):289–299. https://doi.org/10.1080/10408347.2014.949616
Dong Y, Ogawa T, Lin D, Koh HJ, Kamiunten H, Matsuo M, Cheng S (2006) Molecular mapping of quantitative trait loci for zinc toxicity in rice seedling (Oryza sativa L.). Field Crops Res 95(2–3):420–425. https://doi.org/10.1016/j.fcr.2005.03.005
Felker P, Cannell G, Clark PR, Osborn J, Nash P (1983) Biomass production of Prosopis species (mesquite), leucaena, and other leguminous trees grown under heat/drought stress. For Sci 29:592–606. https://doi.org/10.1093/forestscience/29.3.592
Garcia-Franco N, Hobley E, Rico H, Hübner R, Wiesmeier M (2018) Climate-smart soil management in semiarid regions. In: Muñoz MA, Zornoza R (ed) Soil management and climate change; Effects on Organic Carbon, Nitrogen Dynamics, and Greenhouse Gas Emissions. Academic Press, Cambridge, pp 349–368. https://doi.org/10.1016/B978-0-12-812128-3.00023-9
Grigatti M, Boanini E, Cavani L, Ciavatta C, Marzadori C (2015) Phosphorus in digestate-based compost: Chemical speciation and plant-availability. Waste Biomass Valor 6:481–493. https://doi.org/10.1007/s12649-015-9383-2
Heredia B, Tapia R, Young BJ, Hasuoka P, Pacheco P, Roqueiro G (2022) Phytoextraction of Cu, Cd, Zn and As in four shrubs and trees growing on soil contaminated with mining waste. Chemosphere 308:136146. https://doi.org/10.1016/j.chemosphere.2022.136146
Jaiswal S, Shukla P (2020) Alternative strategies for microbial remediation of pollutants via synthetic biology. Front Microbiol 11:808. https://doi.org/10.3389/fmicb.2020.00808
Khan MU, Sessitsch A, Harris M, Fatima K, Imran A, Arslan M, Shabir G, Khan QM, Afzal M (2015) Cr-resistant rhizo- and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front Plant Sci 5:755. https://doi.org/10.3389/fpls.2014.00755
Li R, Zhou Z, Zhang T, Su H, Li J (2023) Overexpression of LSU1 and LSU2 confers cadmium tolerance by manipulating sulfur metabolism in Arabidopsis. Chemosphere 334:139046. https://doi.org/10.1016/j.chemosphere.2023.139046
Marguí E, Hidalgo M, Queralt I (2007) XRF spectrometry for trace elements analysis of vegetation samples. Spectrosc Europe 19(3): 3–17. https://spectroscopyasia.com/system/files/pdf/X-ray_19_3_1.pdf
Mokgalaka-Matlala NS, Flores-Tavizón E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2008) Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis juliflora and P. velutina). Int J Phytoremediat 10(1):47–60. https://doi.org/10.1080/15226510701827069
Muro-González DA, Mussali-Galante P, Valencia-Cuevas L, Flores-Trujillo K, Tovar-Sánchez E (2020) Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environ Sci Pollut Res Int 27:40187–40204. https://doi.org/10.1007/s11356-020-10026-5
Palansooriya KN, Shaheen SM, Chen SS, Tsang DCW, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Enviro Int 134:105046. https://doi.org/10.1016/j.envint.2019.105046
Rai UN, Pandey K, Sinha S, Singh A, Saxena R, Gupta DK (2004) Revegetating fly ash landfills with Prosopis juliflora L.: impact of different amendments and Rhizobium inoculation. Environ Int 30:293–300. https://doi.org/10.1016/S0160-4120(03)00179-X
Ramírez V, Baez A, López P, Bustillos R, Villalobos MA, Carreño R, Contreras JL, Muñoz-Rojas JL, Fuentes-Ramírez LE, Martínez J, Munive JA (2019) Chromium hyper-tolerant Bacillus sp. MH778713 assists phytoremediation of heavy metals by mesquite trees (Prosopis laevigata). Front Microbiol 10:1833. https://doi.org/10.3389/fmicb.2019.01833
Ramírez V, Munive JA, Cortes L, Muñoz-Rojas J, Portillo R, Baez A (2020) Long-chain hydrocarbons (C21, C24, and C31) released by Bacillus sp. MH778713 break dormancy of mesquite seeds subjected to chromium stress. Front Microbiol 11:741. https://doi.org/10.3389/fmicb.2020.00741
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. John Wiley & Sons, New York, pp 193–229
Salt DE, Baxter I, Lahner B (2008) Ionomics and the study of the plant ionome. Ann Rev Plant Biol 59:709–733. https://doi.org/10.1146/annurev.arplant.59.032607.092942
Sarwar N, Saifullah S, Malhi S, Zia MH, Naeem A, Bibi S, Farida G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90(6):925–937. https://doi.org/10.1002/jsfa.3916
Scoccianti V, Iacobucci M, Paoletti MF, Fraternale A, Speranza A (2008) Species-dependent chromium accumulation, lipid peroxidation, and glutathione levels in germinating kiwifruit pollen under Cr(III) and Cr(VI) stress. Chemosphere 73(7):1042–1048. https://doi.org/10.1016/j.chemosphere.2008.07.083
Senthilkumar P, Prince WS, Sivakumar S, Subbhuraam CV (2005) Prosopis juliflora-a green solution to decontaminate heavy metal (Cu and Cd) contaminated soils. Chemosphere 60:1493–1496. https://doi.org/10.1016/j.chemosphere.2005.02.022
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753. https://doi.org/10.1016/j.envint.2005.02.003
Sharma DC, Sharma CP, Tripathi RD (2003) Phototoxic lesions of chromium in maize. Chemosphere 51(1):63–68. https://doi.org/10.1016/S0045-36535(01)00325-3
Singh S, Parihar P, Singh R, Singh V, Prasad S (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Stefanowicz AM, Kapusta P, Zubek S, Stanek M, Woch MW (2020) Soil organic matter prevails over heavy metal pollution and vegetation as a factor shaping soil microbial communities at historical Zn-Pb mining sites. Chemosphere 240:124922. https://doi.org/10.1016/j.chemosphere.2019.124922
Tiodar ED, Văcar CL, Podar D (2021) Phytoremediation and microorganisms-assisted phytoremediation of mercury-contaminated soils: challenges and perspectives. Int J Environ Res Public Health 18(5):2435. https://doi.org/10.3390/ijerph18052435
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462. https://doi.org/10.1016/j.cej.2016.09.029
Usha B, Venkataraman G, Parida A (2009) Heavy metal and abiotic stress inducible metallothionein isoforms from Prosopis juliflora (SW) D.C. show differences in binding to heavy metals in vitro. Mol Genet Genom 281(1):99–108. https://doi.org/10.1007/s00438-008-0398-2
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390. https://doi.org/10.3390/ijms14047370
Watanabe T, Azuma T (2021) Ionomic variation in leaves of 819 plant species growing in the botanical garden of Hokkaido University. Japan J Plant Res 134(2):291–304. https://doi.org/10.1007/s10265-021-01254-y
Watanabe T, Maejima E, Yoshimura T, Urayama M, Yamauchi A, Owadano M, Okada R, Osaki M, Kanayama Y, Shinano T (2016) The ionomic study of vegetable crops. PLoS ONE 11(8):e0160273. https://doi.org/10.1371/journal.pone.0160273
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Scholar Res Notic. https://doi.org/10.5402/2011/402647
Zappala MN, Ellzey JT, Bader J, Peralta-Videa JR, Gardea-Torresdey JL (2014) Effects of copper sulphate on seedlings of Prosopis pubescens (Screwbean Mesquite). Int J Phytoremediat 16(10):1031–1041. https://doi.org/10.1080/15226514.2013.810582
Zhang C, Hiradate S, Kusumoto Y, Morita S, Koyanagi TF, Chu Q, Watanabe T (2021) Ionomic responses of local plant species to natural edaphic mineral variations. Front Plant Sci 12:614613. https://doi.org/10.3389/fpls.2021.614613
Acknowledgements
Financial support was received from Vicerrectoría de Investigación y Estudios de Posgrado (VIEP-BUAP), Instituto de Ciencias (ICUAP), Posgrado en Ciencias (Microbiología) and CONACyT research grant number 252847. Verónica Ramírez and Dolores López are grateful for grant numbers 273291 and 994841 from CONACyT.
Funding
This work was supported by Vicerrectoría de Investigación y Estudios de Posgrado (VIEP-BUAP), Instituto de Ciencias (ICUAP), Posgrado en Ciencias (Microbiología) and CONACyT research grant number 252847. Verónica Ramírez and Dolores López are grateful for grant numbers 273291 and 994841 from CONACyT.
Author information
Authors and Affiliations
Contributions
VR, AB, and JAM conceived, designed, and directed the project, contributed to the interpretation of the results, and designed the figures. VR, DL, VQ, PL, GJ and JM participated in the acquisition of the data and developed the methodologies. AB took the lead in the writing of the manuscript. All authors provided critical feedback, helped to shape the research, discussed the results, and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Maryam Shabani.
Supplementary Information
13762_2023_5297_MOESM1_ESM.jpg
Fig. S1 Wavelength-dispersive X-ray fluorescence diffractogram of P. laevigata seedlings treated for six months with chromium 25 mg/L (A), zinc 3000 mg/L (B), iron 2000 mg/L (C) or nothing (D), in Jensen’s medium.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramírez, V., Lopez, D., Quintero-Hernandez, V. et al. Ionomic analysis of Prosopis laevigata response to heavy metals: phytoremediation potential determined by wavelength-dispersive X-ray fluorescence. Int. J. Environ. Sci. Technol. 21, 4705–4714 (2024). https://doi.org/10.1007/s13762-023-05297-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05297-7