Abstract
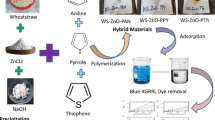
The major source of water pollution is industrial wastewater containing dyes. In the current study, the adsorptive removal of Golden Yellow-XGL dye using green hybrid materials was optimized in the batch approach. The characterization study through SEM, TGA–DSC and XRD showed good porosity, enhanced thermal stability and successful formation of materials while FTIR study confirmed efficient adsorption of dye. The highest adsorption potential for RH (36.9 mg/g), RH–ZnO–PTh (48.9 mg/g), RH–ZnO–PANI (57.8 mg/g) and RH–ZnO–PPy (64.9 mg/g) was obtained at optimized conditions. The adsorption data showed better fitness to pseudo-second-order kinetics and Langmuir sorption isotherm. Thermodynamic study showed exothermic nature of sorption. The mechanism of adsorption was proposed. Desorption and recycling study showed successful reusability of the material up to five cycles. All the results obtained proved these materials to be more efficient, cost effective, ecofriendly, stable and good alternative for dye removal.












Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this article. The raw data retrieved supporting the results of the study are available from the corresponding author on reasonable request.
Abbreviations
- RH:
-
Rice husk
- PANI:
-
Polyaniline
- PPy:
-
Polypyrrole
- PTh:
-
Polythiophene
- ZnO:
-
Zinc oxide
- APS:
-
Ammonium persulfate
- RH–ZnO–PANI:
-
Rice husk–zinc oxide–polyaniline
- RH–ZnO–PPy:
-
Rice husk–zinc oxide–polypyrrole
- RH–ZnO–PTh:
-
Rice husk–zinc oxide–polythiophene
- FTIR:
-
Fourier transform infrared
- SEM:
-
Scanning electron microscope
- TGA:
-
Thermogravimetric analysis
- XRD:
-
X-ray diffraction
- PZC:
-
Point of zero charge
- CTAB:
-
Cetyltrimethylammonium bromide
- SDS:
-
Sodium dodecyl sulfate
- DSC:
-
Differential scanning calorimetry
References
Abbas A, Murtaza S, Shahid K, Munir M, Ayub R, Akber S (2012) Comparative study of adsorptive removal of congo red and brilliant green dyes from water using peanut shell. Middle East J Sci Res 11:828–832
Adegoke KA, Bello OS (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24
Ahmad A, Razali MH, Mamat M, Mehamod FSB, Amin KAM (2017) Adsorption of methyl orange by synthesized and functionalized CNTs with 3-aminopropyltriethoxysilane loaded TiO2 nanocomposites. Chemosphere 168:474–482
Ahmed SM, El-Dib FI, El-Gendy NS, Sayed WM, El-Khodary M (2016) A kinetic study for the removal of anionic sulphonated dye from aqueous solution using nanopolyaniline and baker’s yeast. Arab J Chem 9:1721–1728
Aichour A, Zaghouane-Boudiaf H, Khodja HD (2022) Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: characterization, adsorption properties and reuse studies. Arab J Chem 15:103542
Alaghaa L, Guo L, Ghuzi M, Molatlhegi O, Xub Z (2016) Adsorption of hybrid polyacrylamides on anisotropic kaolinite surfaces: effect of polymer characteristics and solution properties. Colloids Surf A Physicochem Eng Asp 498:285–296
Alipour A, Lakouarj MM (2019) Photocatalytic degradation of RB dye by CdS-decorated nanocomposites based on polyaniline and hydrolyzed pectin: isotherm and kinetic. J Environ Chem Eng 7:102837
Anjum M, Miandad R, Waqas M, Gehany F, Barakat MA (2019) Remediation of wastewater using various nanomaterials. Arab J Chem 12:4897–4919
Ansari R, Mosayebzadeh Z (2011) Application of polyaniline as an efficient and novel adsorbent for azo dyes removal from textile wastewaters. Chem Pap 65:1–8
Anuma S, Mishra P, Bhat BR (2021) Polypyrrole functionalized Cobalt oxide Graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J Hazard Mater 416:125929
Araujo CMBD, Ghislandi MG, Rios AG, Costa GRBD, Nascimento BFD, Ferreira AFP, Sobrinho MADM, Rodrigues AE (2022) Wastewater treatment using recyclable agar-graphene oxide biocomposite hydrogel in batch and fixed-bed adsorption column: bench experiments and modeling for the selective removal of organics. Colloids Surf A Physicochem Eng 639:128357
Asgher M, Bhatti HN (2012) Removal of reactive blue 19 and reactive blue 49 textile dyes by citrus waste biomass from aqueous solution: equilibrium and kinetic study. Can J Chem Eng 90:412–419
Ayad MM, Nasr AAE, Stejskal J (2012) Kinetics and isotherm studies of methylene blue adsorption onto polyaniline nanotubes base/silica composite. Ind Eng Chem 18:1964–1969
Ballav N, Debnath S, Pillay K, Maity A (2015) Efficient removal of reactive black from aqueous solution using polyaniline coated ligno-cellulose composite as a potential adsorbent. J Mol Liq 209:387–396
Bhattacharyya A, Banerjee B, Ghorai S, Rana D, Roy I, Sarkar G, Saha NR, De S, Ghosh TK, Sadhukhan S, Chattopadhyay D (2018) Development of an auto-phase separable and reusable graphene oxidepotato starch based cross-linked bio-composite adsorbent for removal of methylene blue dye. Int J Biol Macromol 116:1037–1048
Bhatti HN, Jabeen A, Iqbal M, Noreen S, Naseem Z (2017) Adsorptive behavior of rice bran-based composites for malachite green dye: isotherm, kinetic and thermodynamic studies. J Mol Liq 237:322–333
Birniwa AH, Mahmud HNME, Abdullahi SS, Habibu S, Jagaba AH, Ibrahim MNM, Ahmad A, Alshammari MB, Parveen T, Umar K (2022) Adsorption behavior of methylene blue cationic dye in aqueous solution using polypyrrole-polyethylenimine nano-adsorbent. Polymers 14:3362
Chaudhary S, Sharma J, Kaith BS, Yadav S, Sharma AK, Goel A (2018) Gum xanthan-psyllium-cl-poly (acrylic acid-co-itaconic acid) based adsorbent for effective removal of cationic and anionic dyes: adsorption isotherms, kinetics and thermodynamic studies. Ecotoxicol Environ Saf 149:150–158
Chen Y, Lin Z, Hao R, Xu H, Huang C (2019a) Rapid adsorption and reductive degradation of naphthol green B from aqueous solution by polypyrrole/attapulgite composites supported nanoscale zero-valent iron. J Hazard Mater 371:8–17
Chen Y, Long W, Xu H (2019b) Efficient removal of acid red 18 from aqueous solution by in-situ polymerization of polypyrrole-chitosan composites. J Mol Liq 287:110–888
Dubey S, Banerjee S, Upadhyay SN, Sharma YC (2017) Application of common nano-materials for removal of selected metallic species from water and wastewaters: a critical review. J Mol Liq 240:656–677
Eldeeb TM, Aigbe UO, Ukhurebor KE, Onyancha RB, El-Nemr MA, Hassaan MA, Osibote OA, Ragab S, Okundaye B, Balogun VA, El-Nemr A (2022) Biosorption of acid brown 14 dye to mandarin-CO-TETA derived from mandarin peels. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02664-1
Elzain AA, Aassar MRE, Hashem FS, Mohamed FM, Ali ASM (2019) Removal of methylene dye using composites of poly (styrene-co-acrylonitrile) nanofibers impregnated with adsorbent materials. J Mol Liq 291:111–335
Gharbani P (2017) Synthesis of polyaniline tin (II) molybdophosphate nanocomposite and application of it in the removal of dyes from aqueous solutions. J Mol Liq 242:229–234
Gouthaman A, Asir JA, Gnanaprakasam A, Sivakumar VM, Thirumarimurugan M, Ahamed MAR, Azarudeen RS (2019) Enhanced dye removal using polymeric nanocomposite through incorporation of Ag doped ZnO nanoparticles: synthesis and characterization. J Hazard Mater 373:493–503
Heybet EN, Ugraskan V, Isik B, Yazici O (2021) Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int J Biol Macromol 193:88–99
Inagaki CS, Oliveira MM, Zarbin AJG (2018) Direct and one-step synthesis of polythiophene/gold nanoparticles thin films through liquid/liquid interfacial polymerization. J Colloid Interface Sci 516:498–510
Jabeen A, Kamran U, Noreen S, Park SJ, Bhatti HN (2022) Mango seed-derived hybrid composites and sodium alginate beads for the efficient uptake of 2,4,6-trichlorophenol from simulated wastewater. Catalysts 12:972
Jadoun S, Chauhan NPS, Chinnam S, Aepuru R, Sathish M, Chundawat NS, Rahdar A (2022) A short review on conducting polymer nanocomposite. Biomed Mater Devices. https://doi.org/10.1007/s44174-022-00009-0
Kharazi P, Rahimi R, Rabbani M (2018) Study on porphyrin/ZnFe2O4 polythiophene nanocomposite as a novel adsorbent and visible light driven photocatalyst for the removal of methylene blue and methyl orange. Mater Res Bull 103:133–141
Kharazi P, Rahimi R, Rabbani M (2019) Copper ferrite-polyaniline nanocomposite: structural, thermal, magnetic and dye adsorption properties. Solid State Sci 93:95–100
Khatamian M, Fazayeli M, Divband B (2014) Preparation, characterization and photocatalytic properties of polythiophene-sensitized zinc oxide hybrid nano composites. Mater Sci Semicond Process 26:540–547
Kumar R, Ahmad R (2011) Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (tgw). Desalination 265:112–118
Lacuesta AC, Herrera MU, Manalo R, Balela MDL (2018) Fabrication of kapok paper-zinc oxide-polyaniline hybrid nanocomposite for methyl orange removal. Surf Coat Technol 350:971–976
Li J, Feng J, Yan W (2013) Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for methylene blue. Appl Surf Sci 279:400–408
Li X, Lu H, Zhang Y, He F (2017) Efficient removal of organic pollutants from aqueous media using newly synthesized polypyrrole/CNTs-CoFe2O4 magnetic nanocomposites. Chem Eng J 2(316):893–902
Lyu W, Li J, Trchova M, Wang G, Liao Y, Bober P, Stejskal J (2022) Fabrication of polyaniline/poly(vinyl alcohol)/montmorillonite hybrid aerogels toward efficient adsorption of organic dye pollutants. J Hazard Mater 435:129004
Ma G, Liang X, Li L, Qiao R, Jiang D, Ding Y, Chen H (2014) Cu-doped zinc oxide and its polythiophene composites: preparation and antibacterial properties. Chemosphere 100:146–151
Maqbool M, Sadaf S, Bhatti HN, Rehmat S, Kausar A, Alissa SA, Iqbal M (2021) Sodium alginate and polypyrrole composites with algal dead biomass for the adsorption of Congo red dye: kinetics, thermodynamics and desorption studies. Surf Interfaces 25:101183
Mashkoor F, Nasar A (2019) Polyaniline/Tectona grandis sawdust: a novel composite for efficient decontamination of synthetically polluted water containing crystal violet dye. Groundw Sustain Dev 8:390–401
Mei JY, Qi P, Wei XN, Zheng XC, Wang Q, Guan XX (2019) Assembly and enhanced elimination performance of 3D graphene aerogel zinc oxide hybrids for methylene blue dye in water. Mater Res Bull 109:141–148
Mendez AS, Curiel JCL, Fuentes GA, Rosales BS, Zarate EM, Rivera VM (2022) Thiophene-based oligomers formed in-situ: a novel sensitizer material of TiO2/HY hybrid material. Top Catal 65:1218–1224
Menezes EWD, Lima EC, Royer B, Souza FED, Santos BDD, Gregorio JR, Costa TMH, Gushikem Y, Benvenutti EV (2012) Ionic silica based hybrid material containing the pyridinium group used as an adsorbent for textile dye. J Colloid Interface Sci 378:10–20
Mishra AK, Agrawal NR, Das I (2017) Synthesis of water dispersible dendritic amino acid modified polythiophenes as highly effective adsorbent for removal of methylene blue. J Environ Chem Eng 5:4923–4936
Mohamed F, Abukhadra MR, Shaban M (2018) Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (PPy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci Total Environ 640–641:352–363
Mu B, Tang J, Zhang L, Wang A (2016) Preparation, characterization and application on dye adsorption of a well-defined two-dimensional superparamagnetic clay/polyaniline/ Fe3O4 nanocomposite. Appl Clay Sci 132–133:7–16
Mustafa M, Bashir S, Moosvi SK, Najar MH, Masoodi MH, Rizvi MA (2022) Hybrid polymer composite of prussian red doped polythiophene for adsorptive wastewater treatment application. Acta Chim Slov 69:7601
Nausheen S, Bhatti HN, Sadaf S, Farrukh Z, Noreen S (2014a) Equilibrium modeling of removal of drimarine yellow hf-3gl dye from aqueous solutions by low cost agricultural waste. J Chem Soc Pak 36:177–190
Nausheen S, Bhatti HN, Farrukh Z, Sadaf S, Noreen S (2014b) Adsorptive removal of Drimarine Red HF-3D dye from aqueous solution using low-cost agricultural waste: batch and column study. Chem Ecol 30:376–392
Noreen S, Bhatti HN, Zuber M, Zahid M, Asgher M (2017) Removal of actacid orange-RL dye using biocomposites: modeling studies. J Environ Stud 26(5):1–10
Noreen S, Bhatti HN, Iqbal M, Hussain F, Sarim FM (2020) Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int J Biol Macromol 147:439–452
Oladipo AA, Gazi M, Saber-Samandari S (2014) Adsorption of anthraquinone dye onto eco-friendly semi-IPN biocomposite hydrogel: equilibrium isotherms, kinetic studies and optimization. J Taiwan Inst Chem Eng 45:653–664
Palanisamy PN, Agalya A, Sivakumar P (2012) Polymer composite—a potential biomaterial for the removal of reactive dye. Electron J Chem 9:1823–1834
Pandimurugan R, Thambidurai S (2016) Synthesis of seaweed-ZnO-PANII hybrid composite for adsorption of methylene blue dye. J Environ Chem Eng 4:1332–1347
Perveen S, Nadeem R, Nosheen F, Tongxiang L, Anwar T (2022) Synthesis of biochar-supported zinc oxide and graphene oxide/zinc oxide nanocomposites to remediate tartrazine dye from aqueous solution using fxed-bed column reactor. Appl Nanosci 12:1491–1505
Rajabi HR, Arjmand H, Kazemdehdashti H, Farsi M (2016) A comparison investigation on photocatalytic activity performance and adsorption efficiency for the removal of cationic dye: quantum dots vs. magnetic nanoparticles. J Environ Chem Eng 4:2830–2840
Rangabhashiyam S, Anu N, Nandagopal MG, Selvaraju N (2014) Relevance of isotherm models in biosorption of pollutants by agricultural by products. J Environ Chem Eng 2:398–414
Rehman R, Raza A, Yasmeen F, Dar A, Al-thagafi ZT, Meraf Z (2022) Recent literature review of significance of polypyrrole and its biocomposites in adsorption of dyes from aqueous solution. Adsorp Sci Technol. https://doi.org/10.1155/2022/7047832
Sa ICD, Silva PMO, Nossol E, Borges PHS, Lepri FG, Semaan FS, Dornellas RM, Pacheco WF (2022) Modified dry bean pod waste (Phaseolus vulgaris) as a biosorbent for fluorescein removal from aqueous media: batch and fixed bed studies. J Hazard Mater 424:127723
Sadaf S, Bhatti HN (2014) Batch and fixed bed column studies for the removal of indosol yellow bg dye by peanut husk. J Taiwan Inst Chem Eng 45:541–553
Sadaf S, Bhatti HN, Nausheen S, Noreen S (2014) Potential use of low-cost lignocellulosic waste for the removal of direct violet 51 from aqueous solution: equilibrium and breakthrough studies. Arch Environ Contam Toxicol 66:557–571
Sadeghnezhad M, Ghorbani M, Nikzad M (2022) Synthesis of magnetic polypyrrole modified sodium alginate nanocomposite with excellent antibacterial properties and optimization of dye removal performance using RSM. Ind Crops Prod 186:115192
Safa Y, Bhatti HN (2011) Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination 272:313–322
Salama A (2017) New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J Colloid Interface Sci 487:348–353
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1–13
Sarojini G, Babu SV, Rajasimman M (2022) Adsorptive potential of iron oxide based nanocomposite for the sequestration of Congo red from aqueous solution. Chemosphere 287:132371
Sawasdee S, Jankerd H, Watcharabundit P (2017) Adsorption of dyestuff in household-scale dyeing onto rice husk. Energy Procedia 138:1159–1164
Stejskal J (2022) Review recent advances in the removal of organic dyes from aqueous media with conducting polymers, polyaniline and polypyrrole, and their composites. Polymers 14:4243
Su CXH, Low LW, Teng TT, Wong YS (2016) Combination and hybridisation of treatments in dye wastewater treatment: a review. J Environ Chem Eng 4:3618–3631
Tahir MA, Bhatti HN, Iqbal M (2016) Solar red and brittle blue direct dyes adsorption onto eucalyptus angophoroides bark: equilibrium, kinetics and thermodynamic studies. J Environ Chem Eng 4:2431–2439
Tanzifi M, Yaraki MT, Karami M, Karimi S, Kiadehi AD, Karimipour K, Wang S (2018) Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J Colloid Interface Sci 519:154–173
Van HT, Nguyen LH, Dang NV, Chao HP, Nguyen QT, Nguyen TH, Nguyen TBL, Thanh DV, Nguyen HD, Thang PQ, Thanh PTH, Hoang VP (2021) The enhancement of reactive red 24 adsorption from aqueous solution using agricultural waste-derived biochar modifed with ZnO nanoparticles. RSC Adv 11:5801–5814
Xu X, Gao BY, Yue QY, Zhong QQ (2010) Preparation and utilization of wheat straw bearing amine groups for the sorption of acid and reactive dyes from aqueous solutions. J Hazard Mater 182:1–9
Yu F, Tian F, Zou H, Ye Z, Peng C, Huang J, Zheng Y, Zhang Y, Yang Y, Wei X, Gao B (2021) ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511
Yunusa U, Ibrahim MB (2019) Reclamation of malachite green-bearing wastewater using desert date seed shell: adsorption isotherms. Desorption Reusability Stud Chemsearch J 10(2):112–122
Zhou J, Lu QF, Luo JJ (2017) Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole based composites. J Clean Prod 167:739–748
Acknowledgements
The authors would like to take this opportunity to express their most profound appreciation to the all participants—the specialist and experts from Department of Chemistry, University of Agriculture, Faisalabad 38000, Pakistan—Ruba Munir, Muhammad Zahid, Raziya Nadeem for Conceptualization, writing—review and editing and methodology; Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad 38000, Pakistan—Tayyaba Samreen for visualization and writing—review and editing, Department of Biochemistry, University of Agriculture, Faisalabad 38000, Pakistan—Saadiya Zia for investigation. Dr. Saima Noreen (corresponding author) is thankful to Higher Education Commission (Indigenous PhD Fellowship for 5000 Scholars HEC Phase-II) for partial purchase of chemicals and equipment being used in this study and the University of Agriculture Faisalabad, Pakistan, for facilities to conduct this research. The valuable support from Lahore University of Management Sciences Lahore (LUMS) and Government College University Faisalabad (GCUF) for characterization of the hybrid materials is highly acknowledged.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, writing—original draft and methodology was performed by TA; formal analysis was contributed by HNB and MA; supervision and conception were contributed by SN. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose, and authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aliyam, T., Noreen, S., Bhatti, H.N. et al. Golden Yellow-XGL dye removal utilizing green hybrid materials: (rice husk–zinc oxide–polythiophene/polyaniline/polypyrrole): batch study. Int. J. Environ. Sci. Technol. 21, 3973–3998 (2024). https://doi.org/10.1007/s13762-023-05258-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05258-0