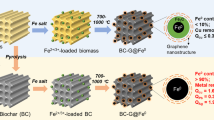
Abstract
The treatment of wastewater is a worldwide concern due its large impact on the environment and human health. Among different types of pollutants, textile effluents (dyes) are increasingly of emerging concern, as they are generally resistant to the conventional treatment methods and harmful to the environment. Herein, biochar-caged zinc oxide (BC-ZnO) and graphene oxide/zinc oxide (BC-GO/ZnO) nanocomposites (NCs) were fabricated and employed in current research for the sorption of tartrazine dye from wastewater. To examine the adsorption ability of BC-ZnO NCs and BC-GO/ZnO NCs, the dynamic fixed-bed column mode sorption experiments were executed under the influence of varied experimental factors, viz. flow rate, initial concentration and bed depth. The column performance was determined as breakthrough curves. Maximum adsorptive removal of tartrazine dye onto BC-ZnO and BC-GO/ZnO NCs was 78.85 ± 0.063 and 88.8 ± 0.31 mg/g, respectively, at optimum conditions, i.e. 1.8 mL/min flow rate, 100 mg/L inlet concentration and 5 cm bed height. Breakthrough curves were analyzed via mathematical models, i.e. Bed Depth Service Time (BDST) and Thomas. Thomas model exhibited good agreement to experimental data with R2 = 0.9981 ± 0.008 indicating validity of the model for the column for BC-GO/ZnO NCs. BC-GO/ZnO NCs was regenerated in five consecutive experiments without a significant loss in process efficiency.







Similar content being viewed by others
References
Abbas R, Hami H, Mahdi N (2019) Removal of doxycycline hyclate by adsorption onto cobalt oxide at three different temperatures: isotherm, thermodynamic and error analysis. Int J Environ Sci Technol 16:5439–5446
Alamdari S, Ghamsari MS, Afarideh H, Mohammadi A, Geranmayeh S, Tafreshi MJ, Ehsani MH (2019) Preparation and characterization of GO-ZnO nanocomposite for UV detection application. Opt Mater 92:243–250
Ali MEM, Assirey EA, Abdel-Moniem SM, Ibrahim HS (2019) Low temperature-calcined TiO 2 for visible light assisted decontamination of 4-nitrophenol and hexavalent chromium from wastewater. Sci Rep 9:1–9
Ali MA, Mubarak MF, Keshawy M, Zayed MA, Ataalla M (2021) Adsorption of tartrazine anionic dye by novel fixed bed Core-Shell-polystyrene Divinylbenzene/Magnetite nanocomposite. Alex Eng J 61:1335–1352
Amina A, Zaghouane-boudiaf H (2019) Highly brilliant green removal from wastewater by mesoporous adsorbents: kinetics, thermodynamics and equilibrium isotherm studies. Microchem J 146:1255–1262
Archana S, Kumar KY, Jayanna B, Olivera S, Anand A, Prashanth M, Muralidhara H (2018) versatile graphene oxide decorated by star shaped zinc oxide nanocomposites with superior adsorption capacity and antimicrobial activity. J Sci Adv Mater Devices 3:167–174
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Aslam S, Sagar RUR, Kumar H, Zhang G, Nosheen F, Namvari M, Mahmood N, Zhang M, Qiu Y (2020) Mixed-dimensional heterostructures of hydrophobic/hydrophilic graphene foam for tunable hydrogen evolution reaction. Chemosphere 245:125607
Ayodele BV, Alsaffar MA, Mustapa SI, Cheng CK, Witoon T (2021) Modeling the effect of process parameters on the photocatalytic degradation of organic pollutants using artificial neural networks. Process Saf Environ Prot 145:120–132
Banerjee S, Joshi S, Mandal T, Halder G (2018) Application of zirconium caged activated biochar alginate beads towards deionization of Cr (VI) laden water in a fixed bed column reactor. J Environ Chem Eng 6:4018–4029
Benally C, Messele SA, El-Din MG (2019) Adsorption of organic matter in oil sands process water (OSPW) by carbon xerogel. Water Res 154:402–411
Bohart G, Adams E (1920) Some aspects of the behavior of charcoal with respect to chlorine. J Am Chem Soc 42:523–544
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712
Chaudhuri SK, Malodia L (2017) Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl Nanosci 7:501–512
Chukwuemeka-Okorie HO, Ekuma FK, Akpomie KG, Nnaji JC, Okereafor AG (2021) Adsorption of tartrazine and sunset yellow anionic dyes onto activated carbon derived from cassava sievate biomass. Appl Water Sci 11:1–8
Cruz JC, Nascimento MA, Amaral HA, Lima DS, Teixeira APC, Lopes RP (2019) Synthesis and characterization of cobalt nanoparticles for application in the removal of textile dye. J Environ Manag 242:220–228
Cruz GJ, Mondal D, Rimaycuna J, Soukup K, Gómez MM, Solis JL, Lang J (2020) Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J Environ Chem Eng 8:103800
Dawood S, Sen TK, Phan C (2018) Performance and dynamic modelling of biochar and kaolin packed bed adsorption column for aqueous phase methylene blue (mb) dye removal. Environ Technol 26:3762–3772
Essandoh M, Garcia RA, Palochik VL, Gayle MR, Liang C (2021) Simultaneous adsorption of acidic and basic dyes onto magnetized polypeptidylated-Hb composites. Sep Purif Technol 255:117701
Garg R, Gupta R, Singh N, Bansal A (2021) Characterization and performance evaluation of synthesized ZnO nanoflowers, nanorods, and their hybrid nanocomposites with graphene oxide for degradation of Orange G. Environ Sci Pollut Res 28:57009–57029
Gonçalves MG, da Silva Veiga PA, Fornari MR, Peralta-Zamora P, Mangrich AS, Silvestri S (2020) Relationship of the physicochemical properties of novel ZnO/biochar composites to their efficiencies in the degradation of sulfamethoxazole and methyl orange. Sci Total Environ 748:141381
Gupta A, Balomajumder C (2016) Simultaneous continuous removal of cr (vi) and phenol from binary synthetic simulated waste water in tea waste packed bed column: Kinetic modeling. J Dispersion Sci Technol 37:656–664
Hethnawi A, Manasrah AD, Vitale G, Nassar NN (2018) Fixed-bed column studies of total organic carbon removal from industrial wastewater by use of diatomite decorated with polyethylenimine-functionalized pyroxene nanoparticles. J Colloid Interface Sci 513:28–42
Huang H, Wang Y, Zhang Y, Niu Z, Li X (2020) Amino-functionalized graphene oxide for Cr (VI), Cu (II), Pb (II) and Cd (II) removal from industrial wastewater. Open Chem 18:97–107
Iqbal MM et al (2021) Effective sequestration of Congo red dye with ZnO/cotton stalks biochar nanocomposite: MODELING, reusability and stability. J Saudi Chem Soc 25:101176
Ismail M, Khan M, Khan SB, Khan MA, Akhtar K, Asiri AM (2018) Green synthesis of plant supported CuAg and CuNi bimetallic nanoparticles in the reduction of nitrophenols and organic dyes for water treatment. J Mol Liq 260:78–91
Ito D, Nishimura K, Miura O (2009) Removal and recycle of phosphate from treated water of sewage plants with zirconium ferrite adsorbent by high gradient magnetic separation. J Phys Conf Ser 156:012033
Jafari-Arvari H, Saei-Dehkordi SS, Farhadian S (2021) Evaluation of interactions between food colorant, tartrazine, and Apo-transferrin using spectroscopic analysis and docking simulation. J Mol Liq 339:116715
Joseph J, Radhakrishnan RC, Johnson JK, Joy SP, Thomas J (2020) Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Mater Chem Phys 242:122488
Karthikeyan P, Banu HAT, Meenakshi S (2019) Removal of phosphate and nitrate ions from aqueous solution using La3+ incorporated chitosan biopolymeric matrix membrane. Int J Biol Macromol 124:492–504
Kausar A, Naeem K, Hussain T, Bhatti HN, Jubeen F, Nazir A, Iqbal M (2019) Preparation and characterization of chitosan/clay composite for direct rose FRN dye removal from aqueous media: comparison of linear and non-linear regression methods. J Mater Res Technol 8:1161–1174
Kosmulski M, Mączka E (2019) Surface charging and points of zero charge of less common oxides: Beryllium oxide. Colloids Surf A 575:140–143
Luo M, Liang Z, Liu C, Qi X, Chen M, Sagar RUR, Yang H, Liang T (2021) Single–atom manganese and nitrogen co-doped graphene as low-cost catalysts for the efficient co oxidation at room temperature. Appl Surf Sci 536:147809
Majumdar S, Baishya A, Mahanta D (2019) Kinetic and equilibrium modeling of anionic dye adsorption on polyaniline emeraldine salt: Batch and fixed bed column studies. Fibers Polym 20:1226–1235
Manoratne C, Rosa S, Kottegoda I (2017) XRD-HTA UV visible, FTIR and SEM interpretation of reduced graphene oxide synthesized from high purity vein graphite. Mater Sci Res India 14:19–30
Melo R, Neto EB, Nunes S, Dantas TC, Neto AD (2018) Removal of Reactive Blue 14 dye using micellar solubilization followed by ionic flocculation of surfactants. Sep Purif Technol 191:161–166
Naeem H, Bhatti HN, Sadaf S, Iqbal M (2017) Uranium Remediation Using Modified Vigna Radiata Waste. Biomass Appl Radiat Isot 123:94–101
Naseem K, Farooqi ZH, Begum R, Irfan A (2018) Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: a review. J Clean Prod 187:296–307
Nasrollahzadeh MS, Hadavifar M, Ghasemi SS, Chamjangali MA (2018) Synthesis of ZnO nanostructure using activated carbon for photocatalytic degradation of methyl orange from aqueous solutions. Appl Water Sci 8:1–12
Nath J, Ray L, Bera D (2016) Continuous removal of malachite green by calcium alginate immobilized Bacillus cereus M116 in packed bed column. Environ Technol Innov 6:132–140
Noorimotlagh Z, Mirzaee SA, Martinez SS, Alavi S, Ahmadi M, Jaafarzadeh N (2019) Adsorption of textile dye in activated carbons prepared from DVD and CD wastes modified with multi-wall carbon nanotubes: Equilibrium isotherms, kinetics and thermodynamic study. Chem Eng Res Des 141:290–301
Ooi GT et al (2018) Biological removal of pharmaceuticals from hospital wastewater in a pilot-scale staged moving bed biofilm reactor (MBBR) utilising nitrifying and denitrifying processes. Bioresour Technol 267:677–687
Ouassif H, Moujahid EM, Lahkale R, Sadik R, Bouragba FZ, Diouri M (2020) Zinc-Aluminum layered double hydroxide: High efficient removal by adsorption of tartrazine dye from aqueous solution. Surf Interfaces 18:100401
Pelalak R et al (2021) Molecular dynamics simulation of novel diamino-functionalized hollow mesosilica spheres for adsorption of dyes from synthetic wastewater. J Mol Liq 322:114812
Qian TT, Wu P, Qin QY, Huang YN, Wang YJ, Zhou DM (2019) Screening of wheat straw biochars for the remediation of soils polluted with Zn (ii) and Cd (ii). J Hazard Mat 362:311–317
Rangabhashiyam S, Suganya E, Selvaraju N (2016) Packed bed column investigation on hexavalent chromium adsorption using activated carbon prepared from Swietenia mahagoni fruit shells. Desalin Water Treat 57:13048–13055
Rehman Sagar RU et al (2020) Large magnetotransport properties in mixed-dimensional van der Waals heterostructures of graphene foam. Carbon 159:648–655. https://doi.org/10.1016/j.carbon.2020.01.001
Russo A, Merlo B, Jacobo S (2021) Adsorption and catalytic degradation of tartrazine in aqueous medium by a Fe-modified zeolite. Clean Eng Technol 4:100211
Sadiq H et al (2021) Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J Mol Liq 335:116567
Sagar RUR et al (2017a) Large, linear, and tunable positive magnetoresistance of mechanically stable graphene foam-toward high-performance magnetic field sensors. ACS Appl Mater Interfaces 9:1891–1898. https://doi.org/10.1021/acsami.6b13044
Sagar RUR, Namvari M, Navale ST, Stadler FJ (2017b) Synthesis of scalable and tunable slightly oxidized graphene via chemical vapor deposition. J Colloid Interface Sci 490:844–849. https://doi.org/10.1016/j.jcis.2016.11.073
Sagar RUR, Galluzzi M, García-Peñas A, Bhat MA, Zhang M, Stadler FJ (2019) Large unsaturated room temperature negative magnetoresistance in graphene foam composite for wearable and flexible magnetoelectronics. Nano Res 12:101–107. https://doi.org/10.1007/s12274-018-2186-6
Sagar RUR et al (2021) Extremely large, linear, and controllable positive magnetoresistance in neodymium-doped graphene foam for magnetic sensors. Mater Today Phys 20:100460. https://doi.org/10.1016/j.mtphys.2021.100460
Sajjadi S-A et al (2019) A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb (II) sorption. J Environ Manag 236:34–44
Salam KA (2019) Assessment of surfactant modified activated carbon for improving water quality. J Encapsul Adsorpt Sci 9:13
Saloglua D, Sahinb OI (2021) Removal of azo dyes–tartrazine, carmoisine, and Allura Red–from wastewater using Spirulina biomass-immobilized alginate beads: equilibrium, kinetics, thermodynamics, desorption, and reusability. Desalin Water Treat 220:431–445
Sampurnam S et al (2019) Synthesis and characterization of Keggin-type polyoxometalate/zirconia nanocomposites—comparison of its photocatalytic activity towards various organic pollutants. J Photochem Photobiol A Chem 370:26–40
Shakya A, Núñez-Delgado A, Agarwal T (2019) Biochar synthesis from sweet lime peel for hexavalent chromium remediation from aqueous solution. J Environ Manag 251:109570
Sharma M, Das P, Datta S, Young AL, Young KL, Liu Y, Willison J, Srivastava S, Shukla AK (2016) Green synthesis of silver–soil nanocomposite from two different sources and its application for the removal of dye solution. Environ Pollut Prot 1:55–68
Tabasum A, Zahid M, Bhatti HN, Asghar M (2018) Fe3O4-GO composite as efficient heterogeneous photo-fenton’s catalyst to degrade pesticides. Mater Res Express 6:015608
Tan X-f et al (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Biores Technol 212:318–333
Tho P et al (2021) Enhanced simultaneous adsorption of As (iii), Cd (ii), Pb (ii) and Cr (vi) ions from aqueous solution using cassava root husk-derived biochar loaded with ZnO nanoparticles. RSC Adv 11:18881–18897
Van HT, Nguyen LH, Nguyen XH, Nguyen TH, Nguyen TV, Vigneswaran S, Rinklebe J, Tran HN (2019) Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: batch and column studies. J Environ Manag 241:535–548
Van HT et al (2021) The enhancement of reactive red 24 adsorption from aqueous solution using agricultural waste-derived biochar modified with ZnO nanoparticles. RSC Adv 11:5801–5814
Wang L, Wang J, He C, Lyu W, Zhang W, Yan W, Yang L (2019) Development of rare earth element doped magnetic biochars with enhanced phosphate adsorption performance. Colloids Surf, A 561:236–243
Yao J, Zhang Y, Dong Z (2021) Enhanced degradation of contaminants of emerging concern by electrochemically activated peroxymonosulfate: performance, mechanism, and influencing factors. Chem Eng J 415:128938
Yu F et al (2021) ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511
Zeb MH et al (2019) Superior magnetoresistance performance of hybrid graphene foam/metal sulfide nanocrystal devices. ACS Appl Mater Interfaces 11:19397–19403. https://doi.org/10.1021/acsami.9b00020
Zhang M, Liu Y-H, Shang Z-R, Hu H-C, Zhang Z-H (2017) Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun 88:39–44
Zhang J, Ge Y, Li Z, Wang Y (2020) Facile fabrication of a low-cost and environmentally friendly inorganic-organic composite membrane for aquatic dye removal. J Environ Manag 256:109969
Zhang Y, Zhao G, Xuan Y, Gan L, Pan M (2021) Enhanced photocatalytic performance for phenol degradation using ZnO modified with nano-biochar derived from cellulose nanocrystals. Cellulose 28:991–1009
Acknowledgements
This work was supported financially by the Special Funds for High Level Research (205200100463), Jiangxi University of Science and Technology and Technology Development Fund (TDF-02-153), Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We have no conflict of interest with any institution or private sector.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perveen, S., Nadeem, R., Nosheen, F. et al. Synthesis of biochar-supported zinc oxide and graphene oxide/zinc oxide nanocomposites to remediate tartrazine dye from aqueous solution using fixed-bed column reactor. Appl Nanosci 12, 1491–1505 (2022). https://doi.org/10.1007/s13204-021-02323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-02323-3