Abstract
In recirculating aquaculture systems, cultivated fish cannot incorporate all the phosphate contained in the feed. Excess phosphate ends up in the culture water and in the sludge produced. If the sludge cannot be valorised directly in agriculture, a chemical recovery as concentrated phosphate is an interesting option to help closing the phosphorus cycle. This study investigates the extent to which accumulated phosphorus can be recovered by acid re-dissolution with subsequent precipitation on sludge from two different recirculating aquaculture systems cultivating African Catfish (Clarias gariepinus). Acid treatment could increase the ratio of dissolved phosphorus available for subsequent precipitation by 53% for extensive and by 61% for intensive fish farming. With the consecutive precipitation in total up to 86% of the phosphorus from the sludge could be recovered. Phosphorus re-dissolution with citric acid requires the highest amount of acid per g dry matter as well as of sodium hydroxide for subsequent precipitation. Sulfuric and nitric acids have comparably lower demands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main objective of today’s intensive agriculture is to generate maximum output while using resources as efficiently as possible (Struik and Kuyper 2017). However, nutrients for crop and livestock production, in particular Nitrogen (N) and Phosphorus (P), are used in excess in order to avoid nutrient undersupply and corresponding growth losses. This issue also applies to the intensive aquaculture. In common practice, only a portion of the supplied nutrients, depending on feeding strategy, feed composition, fish size and temperature is effectively used for the production of aquatic organisms (Cho et al. 1994). (Luo et al. 2010) demonstrated that, depending on the dietary P level, the P retention of yellow catfish P. fulvidraco accounts for a maximum of around 70%. In the study by (Strauch et al. 2018), it is shown that the African Catfish takes up 58–64% of the P contained in the diet, depending on stocking density.
Particularly in intensive aquaculture, loads of nutrients accumulate in the culture water and into the sludges that are deposited (Schneider et al. 2005). In recirculating aquaculture systems (RAS), excess nutrients are discharged via water exchange and filtering units, such as drum filters or sedimentation units, in order to comply with limits for the disposal of culture water and to ensure species-appropriate husbandry conditions (Weirup et al. 2019). However, the state of the art in today’s aquaculture does not always include optimal feed usage which is why high nutrient release and, thus, possible high losses can be assumed. There are various options for using the residual materials (sludge and culture water). One possibility is to spread the sludge on agricultural land (Brod and Øgaard 2021; Burducea et al. 2022). This requires according agricultural demand nearby. Similar to sewage sludge, there is a risk of heavy metals and pharmaceutical substances being released directly into the environment (Kirchmann et al. 2016; Zuloaga et al. 2012). Aquaponics offers another possibility. Here, the remaining nutrients of the culture water can be used on site for crop cultivation (Palm et al. 2018b). The origins of integrated systems for combined crop and fish production are subject to debate and likely have diverse cultural origins. For example, the practice of rice-fish farming in China dates back over 1700 years (Lu and Li 2006). In such systems, fish feed serves as the primary nutrient input, enriching the process water through the excretions of the fish and the decomposition of feed pellets by microorganisms and other organisms. Efficient operation of an aquaponics system hinges on determining the optimal fish-to-plant ratio to maximise nutrient utilisation. A study by (Endut et al. 2010) highlighted that a well-balanced nutrient used in a combined African catfish and water spinach system occurs at a fish feed rate of 15–42 g/m2 of plant production. It’s worth noting that varying nutrient consumption among different crops, coupled with the dependency on feed input and production intensity of the respective fish species, necessitates the evaluation of fish-crop ratios on a case-by-case basis.
Nevertheless, it can be observed that intensified production systems and species that can be reared more intensively, such as the African catfish, generate higher nutrient availability, thereby expanding the potential crop production area. However, in RAS, mechanical filtration techniques play a crucial role in extracting solid particles and enhancing water quality to create favourable conditions for optimal growth rates of the reared fish. Inadequate treatment of effluents can lead to increased oxygen consumption, elevated eutrophication, and blockages in system components such as plumbing and pumps. Furthermore, these particles can cause harm to fish by damaging their gill tissue (Schumann and Brinker 2020; Zhang et al. 2021).
As a result, in aquaponics systems, the liquid phase serves as the primary source of nutrients for crop production, while the concentrated solid waste is typically discarded (Khiari et al. 2019). Research by (Strauch et al. 2018) indicated that approximately 7.1–9.9% of fish feed input remains as solid waste. Additionally, studies conducted by (Cerozi and Fitzsimmons 2017) demonstrated that 25–35% of nutrient-enriched feed contributes to suspended solids in the liquid phase. Consequently, aquaponics systems excel at effectively utilising suspended solids, making them a pivotal technology for advancing the circular economy. However, this principle often doesn’t extend to solid waste, which represents a significant yet largely untapped resource.
If direct material applications are not possible, chemical recovery of the P (and other nutrients) is another option. One opportunity to effectively remove P from the effluent water depicts the application of various precipitants such as iron (II and III) chlorides or alum sulphates producing poorly soluble complexes (Thistleton et al. 2002; Yang et al. 2019). These and further P elimination technologies within the wastewater treatment have a long history mainly to decrease P emissions and the associated eutrophication of surface waters (Sartorius et al. 2012). However, these technologies usually just focus on the removal of dissolved P but not on the further application of the precipitates. (Ylivainio et al. 2021) showed that nutrient-rich residues, which have high iron/alum to P ratio, have a low relative agronomic efficiency and a poor P plant availability.
In recent years, numerous chemical processes have been developed to recover a P rich residue from organic materials. Most of the P-recovery technologies can be divided into two categories: (1) precipitation of dissolved P from culture water or sludge (optionally with targeted enhanced dissolution of the bound P in the sludge) and (2) acid digestion of ash from the incineration of sewage sludge (Sartorius et al. 2012; Spörri et al. 2017; Leinweber et al. 2017). All precipitation processes mainly focus on the transformation of dissolved P into a solid phase and are among the longest practiced P-recovery methods. A straightforward method is employed in the treatment of fermented sludge within the AirPrex process. In the anaerobic environment of the fermenter, P previously captured through biological P removal becomes soluble once again, transitioning into a more dissolved state. This change occurs as a result of the metabolic processes of phosphorus-accumulating organisms (PAO). By outgassing CO2 (stripping or gas suction) the pH in the sludge is increased. Eventually, the addition of magnesium chloride induces the growth of struvite crystals ((NH4)Mg(PO4)·6H2O) which can be extracted by gravity.
Around 90% of the soluble P can be effectively precipitated within a pH range of 7.8–8.2. Importantly, as there is no deliberate effort to induce specific redissolution, the overall P recovery rate from the inflow of the sewage treatment plant typically ranges from approximately 10 to 25%, contingent upon the quantity of recovery reactors employed (Canziani et al. 2023; Bogner and Perduca 2022; Zhou et al. 2019).
This process was mainly developed to reduce natural struvite crystallisation regularly occurring in wastewater treatment plants which often leads to incrustation of reactors and clogged pipes (Everding and Montag 2018). One wet chemical P-recovery method with a targeted dissolution of bound P in sludge is the Gifhorner process also known as the Seaborne process. Sewage sludge or manure are hydrolysed at pH 1.5 by adding sulphuric acid and hydrogen peroxide. Hereafter, magnesium hydroxide is introduced into the P-rich liquid phase. By increasing the pH with sodium hydroxide, both, the crystallisation and precipitation of struvite is finally initiated (Seiler 2014). Due to the high use of chemicals and the resulting economic obstacles, the process was redesigned so that approx. 50% of the P contained can be recovered with the Seaborne process (Seiler 2014). The seaborne process is presently operational both at an industrial scale and within a pilot plant setting. On the industrial plant, at a pH of 3.8 with a reaction time of 1 h, around 66% of the P becomes soluble. Subsequently, a direct iron fixation is carried out at a pH of 5.7 using a 15% Na2S solution to effectively extract metals from the liquid phase. This process not only results in a substantial precipitation of P (approximately 14%), but its advantage lies in yielding an iron-free precipitated product that offers improved availability to plants. Furthermore, within the pH range of 9.3–9.4 an impressive 93% of the dissolved P can be precipitated (Esemen 2012; Hermanussen et al. 2012).
Other processes that work on the similar principle of previous acid leaching and subsequent precipitation are the Stuttgart and Extraphos processes (Ohtake and Tsuneda 2019).
In some European countries, sewage sludge is increasingly incinerated. Since the P in the ash is poorly available to plants, further treatment (2) must be carried out (Krüger et al. 2014). Exemplarily, in Remondis’ TetraPhos process, P is recovered by acid (diluted phosphoric acid) elution from sewage sludge ash of a mono-incinerator. The products of the process are gypsum, concentrated phosphoric acid (basis for fertiliser) and metal salts as possible precipitating salts at wastewater treatment plants. 80% of the P introduced by the ash ends up in the phosphoric acid (Hanßen and Lebek 2016). Another example is the Ash2Phos process developed by Easy Mining, yielding Calcium-Hydroxyapatite.
Transferring the experience from sewage sludge to P-recovery from RAS sludge could become an interesting option, where no direct valorisation, e.g. in Aquaponic systems, is applicable. Depending on the chemical composition of the recyclate, different utilisation pathways are possible, even a direct re-use as feed additive if provided in a digestible form, e.g. as di-calcium-phosphate (CaHPO4). This would require a sufficient P-mobilisation from the solid phase and subsequent precipitation with reasonable demand of chemicals. This led to the overall question of whether chemical P-recovery from RAS sludge is a viable option for the aim to close the P loop in aquaculture. Accordingly, the objectives of this orienting study are
-
i.
Determining the ratio between dissolved P (ready for precipitation) and total P in the raw sludge and the potential of its increase by acid mobilisation,
-
ii.
Investigating the effect of different acids with different adjusted pH values on P-mobilisation with subsequent precipitation and its kinetics and
-
iii.
Deriving a suitable technological process chain for effective P-recovery.
The study was carried out at the end of 2020 at the University of Rostock, Faculty of Agricultural and Environmental Sciences.
Materials and methods
Chemicals
Sulphuric acid (96%, p.a.), nitric acid (≥ 65%, p.a.) and sodium hydroxide (≥ 35%, p.a.) were obtained from Carl Roth GmbH. Citric acid monohydrate granulate was obtained from Brauns-Heitmann GmbH. For P analytics, Ammonium heptamolybdate tetrahydrate, Potassium antimony(III) tartrat and L-Ascorbic acid were purchased from Carl Roth GmbH.
Methodical procedure
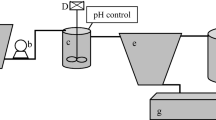
Figure 1 provides a scheme of the applied methodology. Each working step is described in detail, below.
Sampling
Samples for the investigations originate from two aquaculture plants cultivating African Cat fish (C. gariepinus). To receive basic information about the properties of aquaculture sludges, first, samples of the aquaculture research facility Fish Glass House (FGH) at the University of Rostock were analysed in November 2020. Sludge samples were extracted from a sedimentation reactor in a recirculating system with extensive stocking density level (50 kg fish m−3). For the investigations, three independent samples were taken from the bottom of the sedimentation tank after 1 week of sludge accumulation without discharges. The sedimentation tank processes water originating from nine individual tanks, each with a volume of 1.2 m3, accommodating approximately 31 fish per tank. The average water exchange rate in these tanks before the sampling event was 1%. During the 10 days leading up to the sampling, a total of 24.7 kg of feed was provided. For more comprehensive details about the aquaculture facility and its management practices, you can refer to the works of Palm et al. (2018a) and Prüter et al. (2020).
Additionally, three samples were taken from an intensive commercial RAS in Mecklenburg-Western Pomerania (MV), Germany, cultivating African catfish (C. gariepinus) with significantly higher average stocking densities of 200–300 kg fish per cubic meter production volume and a yearly production of 300 MT fish. The production facility is composed of four individual recirculation systems including two sedimentation reactors and a trickling filter for nitrification and CO2 degassing, respectively. Samples of around 60 L were extracted via a pond suction cleaner (Oase Pondovac 4) from two systems.
Both RAS maintain a temperature of approximately 27 °C. The fish feed utilised (Coppens Special pro EF 4.5 mm) comprises 42% crude protein, 13% crude fat, 1.5% crude fibre, and 7.8% ash. This feed also contains 1.9% calcium (Ca) and 1.08% P, and it is employed consistently across both systems.
For further analysis the supernatant was discarded and sub samples were taken from the settled sludge. The sub samples were stored below 5 °C in 20 L PVC containers and processed afterwards within 1 day. Before initiation of each experiment, the sludge was adjusted to ambient temperature. Table 1 shows the parameters of the investigated sludges.
P-Mobilisation procedure
P-recovery via precipitation demands the availability of P as dissolved mineral ion. To develop a suitable wet chemical method for effective P recovery from RAS excess sludge, the initial dissolved P and the P-mobilisation as function of acid, pH and reaction time had to be investigated. For this purpose, a laboratory-based standard procedure was developed to ensure reproducibility of the experiment.
-
(1)
The fresh and pure sludge was analysed for pH (pH meter, WTW) and initial dissolved P-content at 20 °C. The dissolved P was measured after centrifugation for 5 min at 6500 rpm from the supernatant
-
(2)
The dry matter (DM) was analysed as reference value by drying at 105 °C according to DIN EN 12880:2000
-
(3)
The potential and enhanced P-mobilisation was investigated with three different acids. Fresh samples were filled into 85 ml polycarbonate centrifuge tubes and mixed with diluted citric acid (1 mol l−1), nitric acid (1 mol l−1) and sulphuric acid (1 mol l−1), respectively, to adjust different pH between 2 and 7 within each acid group. In order to realise a uniform mobilisation, the samples were mixed on an orbital shaker (Sea Star HS4010A Orbital Shaker) constantly for 1 h at 220 RPM before the dissolved P fraction was analysed after centrifugation (5 min at 6500 rpm) once again according to DIN EN ISO 6878:2004.
-
(4)
P-mobilisation kinetics were investigated with samples of the intensive RAS sludge for each acid at reasonable pH ranges, selected from the above described experiments. The initially dissolved P was measured before acid dosage. Then, the pH was quickly adjusted to the target value and not readjusted over the observation period. After 1–5 h, respectively, the mobilised P concentration was measured. Since the plateau of dissolved P was reached very fast (see results), we repeated the kinetics experiments with denser time steps of ¼ h, ½ h and 1 h of mobilisation.
Measurement methods and calculation of the P mobilisation rate
The rate of P mobilisation was assessed by determining the concentration of P in each fraction. A comparative benchmark was established using the total P content present in the sludge. To extract P from the sludge, microwave (CEM Mars 6) digestion was employed. In this process, 0.5 g of the sample was combined with 10 ml of 65% HNO3. The digestion program, developed through preliminary experiments, involves a 15-min heating phase up to 140 °C, followed by a 30-min plateau phase (max. 850 W), and a subsequent 20-min cooling phase. After dilution, the clear supernatant was subjected to ICP-OES measurement. Additionally, the analysis of dissolved P adhered to the DIN EN ISO 6878:2004 standards. Before analysis, the samples were passed through a glass fibre filter (1–2 µm). This process was replicated three times for each sample, yielding remarkably low standard deviations (Table 1). Considering these consistent outcomes and aiming for practical analytical efficiency, we opted to conduct mobilisation experiments without replicates.
After each process step, P quantities were recalibrated. The quantity of mobilised P pertains to the dissolved P portion within the acid fraction, in relation to the total P within the sludge sample. Likewise, the portion of P precipitated is determined in relation to the total P present in the sludge sample. This is calculated by comparing the measured P in the supernatant post-precipitation with the quantity of P before the precipitation step.
P-Precipitation procedure
Building on the results of the mobilisation, the samples of the intensive RAS were analysed for precipitation properties. (1) Prior to the initiation of the precipitation, all samples except the control were once again mixed with citric (to pH 4.0), sulphuric and nitric (to pH 3.0) acid, respectively, and attached to the orbital shaker for 1 h to mobilise the not solved P fraction. (2) The P concentration of the supernatant after centrifugation was analysed. (3) The pH was measured and then adjusted via sodium hydroxide (1 mol l−1) to pH 7, 8, 9 and 10 within all samples of each acid group. Since in this study we aimed at the precipitation of CaP-compounds, saturated calcium hydroxide solution was added in excess. The precipitation kinetics were analysed in parallel, by sampling at time steps of ½ h, 1 h and 20 h. These samples were analysed for the remaining dissolved P. The efficiency of precipitation was determined by the difference between the dissolved P after mobilisation and that after precipitation.
Results and discussion
Mobilisation
The P-mobilisation as function of pH in both RAS sludge samples is shown in Fig. 2. About 15% (sample A) to 27% (sample B) of the total P content are dissolved and that way directly available for chemical recovery. Starting from this different initial ratio in both samples, there is little difference in P-mobilisation with decreasing pH. At a pH of 4, about 50% of the phosphorus is dissolved, and this proportion increases to a maximum of 75% (sample A), respectively, 85% (sample B) at pH 3. With citric acid, mobilisation at pH values below 4 is somewhat lower compared to nitric or sulfuric acid.
However, a crucial difference between the acids could be detected regarding the specific acid demand, as illustrated in Fig. 3. Below pH 3, the required quantity of the organic acid, C6H8O7, exponentially increases while the acid demand of both inorganic acids continues to increase almost linearly. As a result, for the extensive RAS sludge, the measured acid demand for citric acid to reach pH 2 was six and ten times higher compared to the acid consumption of nitric- and sulphuric acid. Comparing the demand for the same mobilisation efficiency, sulphuric acid performs slightly better than nitric acid. Since, the sharply increasing demand of citric acid to achieve pH values below 3 does not yield in comparably better mobilisation efficiency, its use could be envisaged economically only up to a pH of 3. In contrast, sulphuric acid shows only a weak increase in consumption in the pH range between 2 and 4, so that a fostered P-mobilisation at low pH could be become economically interesting applying this acid.
Mobilisation kinetics
Beside required quantity and price of the acids used, reaction time is a further economically relevant aspect. Accordingly, mobilisation kinetics were investigated exemplarily for the intensive RAS sludges. Based on the results of the pH dependent mobilisation experiments, potentially economic pH ranges were chosen. These differ from acid to acid. For citric acid, a pH range between 3.5 and 4.5 is of interest, while for the mineral acids a range between 3 and 4 was selected. Once the self-determined reaction time (ranging from hourly intervals up to 5 h) elapsed, a sample was promptly collected and the initiation of phase separation followed immediately. This step effectively halted the process of P mobilisation.
With a pKs value of 3.1, citric acid exhibits a moderately robust multi-proton acidity. In stark contrast, the two mineral acids—HNO3 with a pKs value of − 1.3 and H2SO4 with a pKs value of − 3—are characterised as highly potent acids with correspondingly elevated potential for P mobilisation. It’s noteworthy that utilising such intense acids necessitates a higher degree of safety precautions.
Figure 4 show the ratio of dissolved mg P per mg TP for the first 5 h of mobilisation and three different initial pH values; detailed values are listed in Table 2. As expected, the increasing mobilisation efficiency with decreasing pH is visible in the kinetics experiments, too. The dissolved P at time 0 represents the initially dissolved P fraction without acid dosage. The indicated pH0 is the pH value adjusted by the acids used. In the progress of the experiments, the pH value rose slowly and was not readjusted (final Values in Table 2). It can be seen that independent of acid type and pH the ratio between dissolved P and total P increased most in the first hour. In the experiments with low acid addition and higher pH values, no more reaction takes place after the maximum dissolution for the pH value and a plateau phase followed. In the experiments with lower pH values, the mobilisation rate continued to increase, but at a slower rate. Surprisingly in all experiments with nitric acid that the proportion of dissolved P decreased again slightly after 4 h. The same can be seen in experiment 3 with citric acid and experiment 2 with sulphuric acid.
Since the strongest kinetics happened in the first hour, it was worth to regard this period more detailed. Therefore, further the kinetic experiments were repeated with sampling after ¼ h, ½ h and 1 h. The results are shown in Fig. 5. The reaction kinetics of the mineral acids are faster and depending on the chosen pH a reaction time even below 1 h could be envisaged for the mobilisation process, while citric acid requires somewhat longer reaction times.
Precipitation
The P-recovery potential and according technological feasibility depends also on the consecutive precipitation process. The precipitation rate was analysed with regard to required pH and precipitation time. The investigated pH was between 7 and 10 while the overall precipitation time was 20 h with three measuring points (1) 30 min, (2) 1 h and (3) 20 h after initiation of the precipitation reaction. Figure 6 shows the remaining dissolved PO4-Phosphorous for the different, initially adjusted pH values over the reaction time (detailed data are provided in Table 3). As elucidated by Meyer und Weatherall (1982), the transition from amorphous to crystalline calcium phosphate attains its peak transformation point at pH 10.2. In alignment with this principle, the pH levels in this study were systematically adjusted within the spectrum of 7–10, incrementing by steps of 1, while adhering to a maximum permissible deviation of 0.3. The primary objective was to formulate an economically viable strategy that not only boasts exceptional P precipitation efficacy but also curtails the utilisation of alkaline substances.
It becomes obvious that the precipitation reaction is rather fast and strongly pH dependent. For the mineral acids, a high precipitation efficiency is already achieved at pH 8, a nearly complete (> 95%) at pH 10. Effective precipitation of phosphates mobilised by citric acid requires significantly higher pH values (pH|> 10). Also, the precipitation kinetics was faster with mineral acids and completed already after 30 min, while with citric acid, the main precipitation process lasted about 1 h.
Figure 7 shows the demand of sodium hydroxide for pH adjustment. Compared to the acid consumption, the molar demand of hydroxide solution for pH adjustments was considerably slower. The citric acid mobilisate required larger amounts of sodium hydroxide to initiate precipitation and to maintain the desired pH than the inorganic acids.
Discussion
The wet chemical process investigated in this study can be generally highly effective if a far going P recovery from RAS sludges obtained from C. gariepinus is intended. However, many different factors determine the P mobilisation of the particulate P fraction and with that the final recovery potential. Relevant are i.a. the stability of the P compounds, particle size, the type and quantity of the acid used and, consequently, the pH of the solution (Schütze et al. 2020; Wopenka and Pasteris 2005). Overall, the proportion of readily dissolved P to TP, without acid re-dissolution, was between 14.8 and 27.4% in extensive and intensive RAS, respectively. These rather low values are in line with (Venkiteshwaran et al. 2018) who demonstrated for different substrates that an effective P recovery requires a conversion of non-precipitable P to precipitable P. Here, this has been achieved through the use of organic and inorganic acids, whereby the proportion of dissolved P in relation to TP could be increased up to 88%.
The Sankey diagrams in Fig. 8 illustrate for the intensive RAS sludge the different recovery rates without acid digestion compared to digestion with sulphuric acid. At the intensive RAS, 26% of the P can be recovered without targeted dissolution. For the sludge of the extensive plant, the recoverable proportion was only 14%. The reason for this is probably not primarily due to the different feeding regimes but rather to the different initial pH value. When the pH of the sludge from the extensive plant is decreased to a level of pH 5, equivalent to the initial pH of the intensive plant, a parallel recuperation potential ranging between 20 and 30% (contingent upon the specific acid chosen) is attained. This outcome underscores the corollary that a lower pH value yields increased dissolution of P. Still, the mobilisation efficiency remained approximately 10% below those of the sludge from the intensive plant. Applying an acid mobilisation step (here with sulphuric acid), the P recovery can be enhanced to 86% in the intensive plant. At the extensive plant (not illustrated), the mobilisation efficiency is lower and leads accordingly to a lower recovery potential. Here, it can be increased from 17% without targeted redissolution to 71% with prior sulphuric acid treatment. One contributing factor to this phenomenon could be the reduced P load introduced through the feed. Another potential explanation could be variations in the water exchange rate, potentially resulting in a higher proportion of the insoluble P fraction retained relative to the soluble P fraction within the extensive system.
Regardless of the different mobilisation and recovery ratios, the general mobilisation characteristics and mobilisation kinetics of the two investigated sludges follow a similar course. In terms of the P-mobilisation process, for both sludges it was observed that at a plateau phase is reached at about pH 2 irrespective of the acid type used; further acidification would not yield in relevant additional P-mobilisation. The reaction kinetics in both samples showed an asymptotic shape in the first 4 h with a very slow increase after the first hour. The stagnating mobilisation in terms of pH and time indicate that the remaining P is poorly soluble and physico-chemically more firmly bound to the solid phase. In analogy with the results of (Xu et al. 2015), it can be concluded that most of the inorganic P was dissolved, leaving a part of the organic P fraction as a stable, poorly mobilisable fraction.
The organic P fraction may consists of bacterial cell material (e.g. phospholipids or phosphorus proteins) (Committee Report 1970). A similar effect was also observed by McDowell and Sharpley (2002), who examined the P solubility and release kinetics in soil. In their study, they detected that the P solubility and release kinetics is largely dependent on the P compounds and, thus, the type of the substrate. Although comparatively high potential P-recovery rates were obtained with all investigated acids, further investigations could be worthwhile to mobilise the organically bound P as well. This applies namely for residues with higher ratios of organically bound P.
The acid consumption between the citric acid and inorganic acids differed significantly in the lower pH range. This is due to the fact that citric acid is a weak triprotic organic acid with a pKA1 of 3.1 in comparison to the pKA of − 1.37 and − 3.0 of nitric acid and sulphuric acid, respectively. This also explains the steeply increasing acid demand of citric acid to achieve pH values below 3. For that reason, pH 3 was chosen for the precipitation tests for nitric and sulphuric acid while keeping pH 4 for citric acid. However, according to the NFPA (National Fire Protection Association) health hazard rating citric acid is classified as category 1 (slightly hazardous), whereas nitric- and sulphuric acid are classified as category 3 (extreme dangerous) (Global Safety Management Inc. 2014a, b, 2015). This fact is of particular importance from a process engineering perspective, since citric acid could be used with considerably less logistic expenditure on a large scale which consequently leads to time and cost savings. However, it must be realised that the precipitation of CaP-compounds is partially inhibited between pH 7 and pH 9 with previous citric acid mobilisation. This can be caused by two main reactions: (1) citrate as a product of the citric acid dissociation reacts with the hydrated Ca2+-Ions and forms calcium-citrate-crystals and (2) the COOH group of the citric acid dissociates at lower pH values and reacts with the Ca2+-ions resulting in a complexation of citrate with Ca2+ and an inhibition of CaP crystal growth (Berg and Tiselius 1989; Grossl and Inskeep 1991, 1992). As a result, higher quantities of sodium hydroxide and calcium will be needed to increase the pH and maximise the P-recovery efficiency.
Apart from that, precipitation rates provided very good results. The precipitation of the mobilised P led to high efficiencies of 96–98% at reasonable short reaction times (0.5 to 1 h). In order to achieve an efficient large-scale implementation with high phosphorus yields, several parameters need to be considered.
Depending on the acid used a pH range between 2 and 3 and above 3 for the respective inorganic and organic acids should be adjusted to guarantee both, high mobilisation efficiencies and moderate acid consumptions of around 60–70% and 5 mmol g−1 dry matter. For a successful P-recovery, a moderate pH increases to 8–9 is already highly effective for the mineral acids. With citric acid, the pH should be raised to pH 10. In this regard, it is recommended to keep the adjusted pH for the initiation of the precipitation within the limits for an overall base consumption below 0.3 mol l−1 mobilised sludge. Applying these suggestions, a phosphorus yield of 70–80% can be expected.
The primary advantage of RAS over other farming methods lies in their comprehensive environmental control. RAS offers the capability to maintain highly stable species-specific cultivation conditions, a significant contrast to flow-through or net-pen systems, which heavily rely on ambient environmental factors. The reduced reliance on surrounding conditions results in lower frequencies of infections and pathogen pressure, subsequently leading to decreased usage of pharmaceuticals (Meisch und Stark 2019). Furthermore, certain pharmaceuticals, especially antibiotics, can adversely affect the cultivation environment. The pivotal role of microorganisms in RAS involves transforming harmful chemical substances like ammonia and nitrite into less detrimental forms. The use of antibiotics can disrupt this microbiome equilibrium (Silva et al. 2021). In the European Union, various antibiotics, such as tetracyclines and parasiticides, are prohibited in aquaculture due to concerns over antibiotic resistance and alterations in microbial communities (Bondad-Reantaso et al. 2023). Consequently, aquaculture considerably reduces pharmaceutical consumption. While complete avoidance of pharmaceuticals is ideal to prevent environmental impact, situations may necessitate their use, in which case effective downstream purification stages should be implemented. A photodegradation-based treatment, extensively described by Silva et al. (2021), is one such example.
Heavy metals represent another factor that can hinder the utilisation of RAS sludges. The introduction of heavy metals into fish farms primarily occurs through fertilisers, feed, surface water runoff, and agricultural wastewater. These metals accumulate in both fish and the sediments of rivers or within fish farm sludge (Emenike et al. 2021). However, heavy metal contamination varies considerably between different RAS systems. Research indicates that salmon manure and African catfish sludge generally exhibit low heavy metal content, rendering them suitable for agricultural soil fertilisation (Campo et al. 2010; Gebauer und Eikebrokk 2006).
Common routes for heavy metal influx into RAS include feed ingredients and fertilisers (Boyd und Massaut 1999; Nyamete et al. 2020). It’s important to note that the potential applications of sludge can significantly differ based on the specific system, underlining the necessity of heavy metal content analysis prior to utilisation. In instances where heavy metal content exceeds national fertiliser usage limits, removal techniques should be employed. Various effective methods for heavy metal extraction from wastewater have been reported, including adsorption, membrane-based treatments, chemical processes, electrical approaches, and photocatalysis (Qasem et al. 2021). However, the consider potential generation of toxic waste by certain methods, necessitating further treatment.
In-depth examination of heavy metal and pharmaceutical substance dynamics throughout the developed process warrants further investigation and exploration in subsequent studies.
Further studies should focus on the application of organic acids for the P-recovery process, due to the easier logistics. Regarding citric acid, the inhibition of CaP-precipitation below pH 10 should be better enlighted and/or different precipitation partners could be envisaged. Also, other acids, easily manageable under intensive conditions, should be investigated. The precipitation kinetics was investigated, here, only to estimate the required reaction time in technical process. (Rahaman et al. 2008) conducted comparable investigations for struvite precipitation. They could demonstrate that, the precipitation was terminated after approximately 50–250 min depending on the Mg:P ratio. The CaP precipitation occurs obviously fast; for an economic reactor design it could be worthwhile to perform experiments with even shorter precipitation times < 30 min. From an aquaculture perspective, it could be demonstrated that the implementation of a P recovery process could retain most of the feeded P in a concentrated and flexibly applicable mineral form. (Prüter et al. 2020) identified calcium phosphates as the dominant P compound at the FGH research system and suspect that this is also the case for other plants with limy water. Calcium phosphates are also the dominant form of bound P in the Baltic Sea (Prüter et al. 2019). Under anaerobic conditions, these P-sinks can release large amounts of phosphorus through reduction and microbial decomposition (Zinder and Stumm 1985; Boyd 2015). This process is well known in the Baltic Sea (Conley et al. 2002). Oxygen availability in the Baltic Sea is very variable, which often leads to a dissolution of the P compounds from the sediment (Nausch et al. 2005). P recovery can thus reduce the eutrophication potential of aquaculture facilities and further reduce the impact of aquaculture facilities on the environment.
Conclusion
The recovery and recycling of P, as a eutrophic and critical element, is also relevant in aquaculture. The incomplete uptake of P by the fish leads to P deposition in the sludge. With the wet-chemical process investigated here, an effective and applicable technology for P recovery could be demonstrated. For the investigated African Catfish RAS the study’s key messages are:
-
Effective chemical P recovery from RAS sludge requires a mobilisation step
-
Lower initial pH values in the RAS improves mobilisation and leads to lower acid demand
-
Sulphuric, nitric and citric acids can achieve recovery rates between 71 and 86% of the P content in the sludge
-
High mobilisation rates occur in the first hour, precipitation is largely completed after 30 min
-
Various feeding regimes do not lead to significant differences in the P content of the sludge (mg P/g DM)
P recovery can reduce the eutrophication potential of aquaculture systems and further reduce the impact of aquaculture systems on the environment. If this additional effort is worthwhile has to be weighed up with regard to other parameters for the specific application. Further studies with different sludges, ideally from the production of different aquaculture species, need to be analysed to develop a universal approach for an efficient implementation of the developed method. Furthermore, economic evaluations are needed to define parameters such as the type of acid, the amount of acid and the pH value in order to estimate the actual mobilisation rates and carry out a viable, cost-effective P recovery. The objective of the present study was to identify and formulate an effective method for P extraction from aquaculture sludges at a laboratory scale. The practical viability of the developed process, which has been successfully tested in laboratory settings, is notably high. This can be attributed to the process’s straightforward steps, the uncomplicated nature of the additives—especially citric acid, which demonstrates remarkable versatility—and the modular design of the containers.
The utilisation of chemicals such as citric acid offers distinct advantages: (1) its widespread availability in the global market, (2) its cost-effectiveness compared to inorganic acids, and (3) its low potential for hazards.
While the current investigation showcases promising outcomes, the implementation and adaptability of various methods at a larger scale necessitate further exploration. The method under examination holds significant promise to deliver favourable outcomes even within rudimentary conditions, taking into account constraints in resource availability and educational levels.
References
Berg C, Tiselius H-G (1989) The effects of citrate on hydroxyapatite induced calcium oxalate crystallization and on the formation of calcium phosphate crystals. Urol Res 17:167–172
Bogner Rudolf, Perduca Davide (2022) Controlles phosphate precipitation—significant optimisation. Potential in enhanced biological phosphorus removal sludge treatment applying the AIRPREX®-procedure. Proc Environ Sci Eng Manag 4:869–874
Boyd Claude E (2015) Water quality. An introduction, 2nd edn. Springer, Berlin
Brod E, Øgaard AF (2021) Closing global P cycles: the effect of dewatered fish sludge and manure solids as P fertiliser. Waste Manag 135:190–198. https://doi.org/10.1016/J.WASMAN.2021.08.041
Burducea M, Lobiuc A, Dirvariu L, Oprea E, Olaru M, Stefan T, Ciprian G, Stoleru V, Poghirc VA, Cara IG, Filip M, Rusu M, Zheljazkov VD, Barbacariu CA (2022) Assessment of the fertilization capacity of the aquaculture sediment for wheat grass as sustainable alternative use. Plants. https://doi.org/10.3390/PLANTS11050634
Canziani R, Boniardi G, Turolla A (2023) Phosphorus recovery—recent developments and case studies. In: Prasad MNV, Smol M (eds) Sustainable and circular management of resources and waste towards a green deal. Elsevier, Amsterdam, pp 269–281
Cerozi BS, Fitzsimmons K (2017) Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric Syst 153:94–100. https://doi.org/10.1016/j.agsy.2017.01.020
Cho CY, Hynes JD, Wood KR, Yoshida HK (1994) Development of high-nutrient-dense, low-pollution diets and prediction of aquaculture wastes using biological approaches. Aquaculture 124(1–4):293–305
Conley DJ, Humborg C, Rahm L, Savchuk OP, Wulff F (2002) Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ Sci Technol 36(24):5315–5320. https://doi.org/10.1021/ES025763W
Endut A, Jusoh A, Ali N, Wan Nik WB, Hassan A (2010) A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Biores Technol 101(5):1511–1517. https://doi.org/10.1016/j.biortech.2009.09.040
Esemen T (2012) Untersuchungen zur technischen und wirtschaftlichen Optimierung der Nährstoffrückgewinnung aus Klärschlamm. Institut für Siedlungswasserwirtschaft. https://doi.org/10.24355/dbbs.084-202103230742-0
Everding W, Montag D (2018) A1 Modulbeschreibungen Phosphorrückgewinnung-AirPrex®. Abschlussbericht E-Klär.
Global Safety Management Inc. (2014a). Safety Data Sheet Citric Acid,Anhydrous 2014. https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-c/S25255.pdf
Global Safety Management Inc. (2014b). Safety Data Sheet Nitric Acid, 0.1M 2014. https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-n/S25859.pdf
Grossl PR, Inskeep WP (1991) Precipitation of dicalcium phosphate dihydrate in the presence of organic acids. Soil Sci Soc Am J. https://doi.org/10.2136/sssaj1991.03615995005500030006x
Grossl PR, Inskeep WP (1992) Kinetics of octacalcium phosphate crystal growth in the presence of organic acids. Geochimica et Cosmochimica Acta. https://doi.org/10.1016/0016-7037(92)90322-A
Hanßen H, Lebek M (2016) Phosphorrecycling aus Klärschlammasche in Hamburg. Rohstoffgewinnung von Hamburg Wasser nach dem TetraPhos®-Verfahren. Korrespondenz Abwasser 42:886–892
Hermanussen O, Müller-Schaper J, Haun E, Weichgrebe D, Rosenwinkel K-H, Esemen T, Dockhorn T, Dichtl N (2012). Wissenschaftliche Begleitung der großtechnischen Anwendung der Seaborne-Technologie auf der KA Gifhorn. Zusammenfassung der durchgeführten Untersuchungen und technisch-wirtschaftliche Bewertung der Verfahrenstechnik. https://docplayer.org/storage/58/42430184/1691577842/k7GSl8PKkYtwD7k6Pijy5A/42430184.pdf. Accessed 1 Aug 2023
Global Safety Management Inc (2015) Safety Data Sheet Sulfuric Acid, 3M, 1–7. https://www.fishersci.com/store/msds?partNumber=S25899&productDescription=sulfuric-acid-solutions-various&vendorId=VN00115888&keyword=true&countryCode=US&language=en.
Khiari Z, Kaluthota S, Savidov N (2019) Aerobic bioconversion of aquaculture solid waste into liquid fertilizer: effects of bioprocess parameters on kinetics of nitrogen mineralization. Aquaculture 500:492–499. https://doi.org/10.1016/j.aquaculture.2018.10.059
Kirchmann H, Börjesson G, Kätterer T, Cohen Y (2016) From agricultural use of sewage sludge to nutrient extraction: a soil science outlook. Ambio. https://doi.org/10.1007/s13280-016-0816-3
Krüger O, Grabner A, Adam C (2014) Complete survey of German sewage sludge ash. Environ Sci Technol 48(20):11811–11818. https://doi.org/10.1021/ES502766X
Leinweber P, Bathmann U, Buczko U, Douhaire C, Eichler-Löbermann B, Frossard E, Ekardt F, Jarvie H, Krämer I, Kabbe C, Lennartz B, Mellander PE, Nausch G, Ohtake H, Tränckner J (2017) Handling the phosphorus paradox in agriculture and natural ecosystems: Scarcity, necessity, and burden of P. Ambio 47(1):3–19. https://doi.org/10.1007/S13280-017-0968-9
Lu J, Li X (2006) Review of rice–fish-farming systems in China—One of the globally important ingenious agricultural heritage systems (GIAHS). Aquaculture 260(1–4):106–113. https://doi.org/10.1016/j.aquaculture.2006.05.059
Luo Z, Tan XY, Liu X, Wang WM (2010) Dietary total phosphorus requirement of juvenile yellow catfish Pelteobagrus fulvidraco. Aquacult Int 18(5):897–908. https://doi.org/10.1007/s10499-009-9310-2
McDowell RW, Sharpley AN (2002) Phosphorus solubility and release kinetics as a function of soil test P concentration. Geoderma 112(1–2):143–154. https://doi.org/10.1016/S0016-7061(02)00301-4
Nausch G, Feistel Nagel R, Ulrich Lass H, Nagel K, Siegel H (2005). Hydrographisch-chemische Zustandseinschätzung der Ostsee 2004. https://www.io-warnemuende.de/files/forschung/meereswissenschaftliche-berichte/mebe62_2004-zustand-hc.pdf. Accessed 17 April 2023
Ohtake H, Tsuneda S (eds) (2019) Phosphorus recovery and recycling. Singapore, Springer
Palm HW, Knaus U, Wasenitz B, Bischoff AA, Strauch SM (2018a) Proportional up scaling of African catfish (Clarias gariepinus Burchell, 1822) commercial recirculating aquaculture systems disproportionally affects nutrient dynamics. Aquaculture 491:155–168. https://doi.org/10.1016/j.aquaculture.2018.03.021
Palm HW, Knaus U, Appelbaum S, Goddek S, Strauch SM, Vermeulen T, Jijakli MH, Kotzen B (2018b) Towards commercial aquaponics: a review of systems, designs, scales and nomenclature. Aquacult Int 26(3):813–842. https://doi.org/10.1007/S10499-018-0249-Z/TABLES/3
Prüter J, Leipe T, Michalik D, Klysubun W, Leinweber P (2019) Phosphorus speciation in sediments from the Baltic Sea, evaluated by a multi-method approach. J Soils Sedim. https://doi.org/10.1007/s11368-019-02518-w
Prüter J, Strauch SM, Wenzel LC, Klysubun W, Palm HW, Leinweber P (2020) Organic matter composition and phosphorus Speciation of Solid Waste from an African Catfish recirculating aquaculture system. Agriculture 10(10):466. https://doi.org/10.3390/agriculture10100466
Rahaman MS, Ellis N, Mavinic DS (2008) Effects of various process parameters on struvite precipitation kinetics and subsequent determination of rate constants. Water Sci Technol 57(5):647–654. https://doi.org/10.2166/wst.2008.022
Report C (1970) Chemistry of nitrogen an phosphorus in water. J Am Water Works Assoc 62(2):127–140
Sartorius C, von Horn J, Tettenborn F (2012) Phosphorus recovery from wastewater-expert survey on present use and future potential. Water Environ Res 84(4):313–322. https://doi.org/10.2175/106143012x13347678384440
Schneider O, Sereti V, Eding EH, Verreth JAJ (2005) Analysis of nutrient flows in integrated intensive aquaculture systems. Aquacult Eng. https://doi.org/10.1016/j.aquaeng.2004.09.001
Schumann M, Brinker A (2020) Understanding and managing suspended solids in intensive salmonid aquaculture: a review. Rev Aquac 12(4):2109–2139. https://doi.org/10.1111/raq.12425
Schütze E, Gypser S, Freese D (2020) Kinetics of phosphorus release from vivianite, hydroxyapatite, and bone char influenced by organic and inorganic compounds. Soil Syst 4(1):1–20. https://doi.org/10.3390/soilsystems4010015
Seiler U (2014) Vergleich von Verfahren zur Phosphatgewinnung aus Abwasser und Klärschlämmen. Energie aus Abfall 11:731–747
Spörri A, Erny I, Hermann L, Hermann R (2017) Beurteilung von Technologien zur Phosphor-Rückgewinnung, pp 1–63
Strauch SM, Wenzel LC, Bischoff A, Dellwig O, Klein J, Schüch A, Wasenitz B, Palm HW (2018) Commercial African Catfish (Clarias gariepinus) recirculating aquaculture systems: assessment of element and energy pathways with special focus on the phosphorus cycle. Sustainability. https://doi.org/10.3390/su10061805
Struik PC, Kuyper TW (2017) Sustainable intensification in agriculture: the richer shade of green. A review. Agron Sustain Dev. https://doi.org/10.1007/S13593-017-0445-7
Thistleton J, Berry TA, Pearce P, Parsons SA (2002) Mechanisms of chemical phosphorus removal II Iron(III) salts. Process Saf Environ Protect Trans Inst Chem Eng Part B. https://doi.org/10.1205/095758202762277623
Venkiteshwaran K, McNamara PJ, Mayer BK (2018) Meta-analysis of non-reactive phosphorus in water, wastewater, and sludge, and strategies to convert it for enhanced phosphorus removal and recovery. Sci Total Environ 644:661–674. https://doi.org/10.1016/J.SCITOTENV.2018.06.369
Weirup L, Dammshäuser A, Hamer H, Reitner A, Meyer S (2019) Machbarkeitsstudie zur Verwendung von salzhaltigem Grundwasser für die kreislaufbasierte Aquakultur in Schleswig-Holstein. Büsum, Deutschland, Austen, G. (Hrsg)
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25(2):131–143. https://doi.org/10.1016/j.msec.2005.01.008
Xu Y, Hu H, Liu J, Luo J, Qian G, Wang A (2015) pH dependent phosphorus release from waste activated sludge: contributions of phosphorus speciation. Chem Eng J 267:260–265. https://doi.org/10.1016/J.CEJ.2015.01.037
Yang F, Zhang S, Song J, Du Q, Li G, Tarakina NV, Antonietti M (2019) Synthetic humic acids solubilize otherwise insoluble phosphates to improve soil fertility. Angewandte Chem Int Ed. https://doi.org/10.1002/anie.201911060
Ylivainio Kari, Lehti Alma, Jermakka Johannes, Wikberg Hanne, Turtola Eila (2021) Predicting relative agronomic efficiency of phosphorus-rich organic residues. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.145618
Zhang H, Gao Y, Liu J, Lin Z, Lee T, Chew H, Haslenda Wu, Wei-Min Li, Lia C (2021) Recovery of nutrients from fish sludge as liquid fertilizer to enhance sustainability of aquaponics: a review. Chem Eng Trans 83:55–60. https://doi.org/10.3303/CET2183010
Zhou K, Remy C, Kabbe C, Barjenbruch M (2019) Comparative environmental life cycle assessment of phosphorus recovery with different generations of the AirPrex® systems. Int J Environ Sci Technol 16(5):2427–2440. https://doi.org/10.1007/s13762-018-1881-x
Zinder B, Stumm W (1985) Die Auflösung von Eisen(111)-oxiden; ihre Bedeutung im See und im Boden. Separatdruck aus Chimia 39(9):280–288
Zuloaga O, Navarro P, Bizkarguenaga E, Iparraguirre A, Vallejo A, Olivares M, Prieto A (2012) Overview of extraction, clean-up and detection techniques for the determination of organic pollutants in sewage sludge: a review. Anal Chim Acta 736:7–29. https://doi.org/10.1016/J.ACA.2012.05.016
Acknowledgements
This research was funded by the Leibniz Association within the scope of the Leibniz ScienceCampus Phosphorus Research Rostock (www.sciencecampus-rostock.de).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Maryam Shabani.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schleyken, J., Gumpert, F., Tränckner, S. et al. Enhanced chemical recovery of phosphorus from residues of recirculating aquaculture systems (RAS). Int. J. Environ. Sci. Technol. 21, 3775–3788 (2024). https://doi.org/10.1007/s13762-023-05226-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05226-8