Abstract
Radioactivity measurements for water, sediment, microbial films and the bioaccumulation of radionuclides by extremophiles from hypersaline lakes and hot springs were carried out as scarcity studies in the Siwa Oasis, Egypt. Natural and man-made radionuclides were measured using high-resolution γ-spectrometry. Different radionuclides behaved differently in different environmental samples, while radionuclides were higher in microbial films compared to sediment, but all radionuclide levels except 226Ra in water were generally low. Microbial films from hypersaline lakes had higher concentrations of 40K, while microbial films from freshwater hot springs had the highest concentrations of 226Ra, 232Th and 137Cs. The calculated radiological hazard index parameters of radium equivalent activity (Raeq), absorbed dose rate (D), annual effective dose (AED) and external hazard (Hex) in the sediment were within acceptable limits, but were higher in the microbial film samples. Otherwise, the potential cancer risk of the three freshwater springs was 0.00244 ± 0.000293, 0.00135.6 ± 0.000172 and 0.00155.2 ± 0.000198. In addition, the bioaccumulation factor for microbial films indicated that they are good accumulators of radionuclides, especially for 226Ra and 232Th, which may contribute to their effectiveness in removing radionuclides from ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioecology is an interdisciplinary science concerned with understanding and quantifying the behaviour of radionuclides in the environment and the processes that determine their transport through ecosystems to many different types of receptors such as plants, animals and humans. A second aspect of this science is the assessment of radiological dose and impact on humans and their environment from the present, past or future. The behaviour of radionuclides in water is central to their migration because water is one of the main factors in the movement and distribution of elements on Earth. Therefore, aquatic ecosystems strongly influence and are influenced by the fate of radionuclides (Khater 1998). In particular, the natural decay series of radionuclides 238U, 232Th and 40K are responsible for the radioactivity in groundwater. 226Ra is one of the most important elements in the 238U series, indicating the abundance of uranium in the environment. The long half-life of 226Ra, which decays to radon (222Rn), makes 226Ra a Class A carcinogen and is the second most common cause of lung cancer (EPA 2006). The concentration of radionuclides in groundwater is discovered by the mineralogical composition of the aquifer and by the geochemical and hydrochemical conditions, which may change over time as groundwater is degraded (Schubert et al. 2011). Groundwater increases strongly in radioactivity as it passes through fractures in the bedrock (Canu et al. 2011). Environmental measurements of radioactivity levels in groundwater sources are necessary to protect residents from toxic and high exposure doses through ingestion (Yuce et al. 2009).
Siwa Oasis is a deep natural depression, approximately 23 m below sea level. Agriculture is the most popular activity in the Siwa Oasis, which is dependent on groundwater from both dug wells and naturally flowing springs (Elkaffas et al. 2013). The lakes of Siwa are the watersheds of the discharged water from the cultivated areas, natural springs and flowing wells (Dahab 2004; El-Sayed et al. 2017). The non-renewable Nubian sandstone aquifer in the Siwa Oasis is the only water resource available for a variety of uses in the area (Ebraheem et al. 2003; Gossel et al. 2004). This aquifer is suffering from water depletion mainly due to excessive extraction from dug wells (Gad et al. 2018). The geochemical properties of the aquifer, especially the toxic metals and radionuclides, are critical in the management of aquifer use and health problems (Baba and Tayfur 2011; Murad et al. 2014; Yehia et al. 2017). Elevated levels of radioactive materials and daughter products may be found in the groundwater of areas rich in radionuclides. The presence of radionuclides with high radiotoxicity in water and the associated health risks requires special attention (Nriagu et al. 2012; Khodashenas et al. 2012). There is a strong need to develop a systematic programme for monitoring radioactive contaminant systems to protect humans and the environment due to the rapidly increasing dependence on groundwater as the sole water supply in such areas (Walencik et al. 2010).
The transfer of radionuclides to the environment must be considered in the initial stages of an environmental impact assessment. Assessment of radionuclide uptake by marine biota depends on the use of concentration factors (Brown et al. 2004). Microorganisms, such as microbial that have witnessed and survived various extreme conditions on Earth over time, could be used to remediate radioactive contamination (Gazso 2001). Microbes in radionuclide environments have mechanisms for their survival and radiation tolerance, focusing on the interactions between bacterial cells and radionuclides, which have immense potential for bioremediation (Shukla et al. 2017). Various algae, including macroalgae, are highly resistant to metals and U, which can be isolated at very high concentrations. As a result, macrophytes and macroalgae have been used as biomonitoring systems for radionuclide contamination in the marine environment (Strezov and Nonova 2005; Al-Masri et al. 2003; Zalewska and Saniewski 2011). In view of this, the study of radioactivity levels in algae is useful as a bioindicator of contamination of ecological communities and the bioremediation process (Garza 2005).
In the extremophilic groundwater, ecosystem of Siwa Oasis grows a special density of cyanobacteria and bacteria embedded in polysaccharide secretions (cyanobacteria–bacteria mat). This extremophilic cyanobacterial–bacterial mat grows in extreme habitats with high temperatures (> 50 °C) and hypersaline water (200%). These films include cyanobacteria, oxygenic photosynthetic bacteria, aerobic heterotrophs and anaerobes, sulphur-reducing bacteria, sulphur-oxidising bacteria and methanogenic archaea, among other biofunctional classes. Adaptations, competition or synergistic cooperation among these organisms result in unique physiological behaviour (El Karim and Goher 2016) and cause them to possess a large number of active sites and more than twenty active functional groups (e.g. hydroxyl, carboxyl, phenol, carboxyl, phosphorus and sulphydryl) that enhance metal adsorption (Al-Qahtani et al. 2021). These extremophilic microbial films efficiently remove Zn+2, Fe+2 and Cd+2 (Ali et al. 2019), Cu+2, Ni+2 and Pb+2 (Al-Qahtani et al. 2021) and hexavalent Cr and Mo (Abdelakrim et al., in press) from aqueous solution. The ability of microbial films to accumulate radionuclides has not been investigated in this area. It is, therefore, of particular interest to know the extent to which these microbial mats may be capable of radionuclide uptake and accumulation. Therefore, the aim of this study was to assess the radioactivity levels in the groundwater, sediment and microbial mats, to determine the bioaccumulation factor in order to follow-up the potential use of cyanobacterial–bacterial mats as bioindicators and bioacumulators of radionuclides and, furthermore, to estimate the radiation hazard parameters and cancer risk of this area.
Materials and methods
Study area
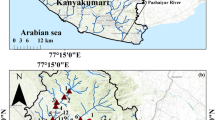
The Siwa Oasis is located in Egypt’s Western Desert, 120-km east of the Egyptian–Libyan border and 300-km south of the Mediterranean Sea (Fig. 1). The map was created using ArcGIS Pro 3.1® software from Esri. ArcGIS® and ArcMapTM are the intellectual property of Esri and are used here under licence and copyright © Esri. All rights reserved. For more information about Esri® software, visit www.esri.com. The oasis extends approximately 23 m below sea level in an east–west direction (Gindy and El-Askary 1969). The Siwa depression has an abundance of naturally flowing groundwater (Masoud and Koike 2006). There are two main aquifers; the shallowest (upper Siwa) and the deepest (Nubian sandstone). Springs are present in the shallow Middle Miocene carbonates, which are completely isolated from the Nubian sandstone (the deep aquifer) (Masoud and Koike 2006).
Sampling and sample preparation
The samples were collected from three freshwater springs and three hypersaline lakes in the Siwa Oasis in January 2018 (Fig. 1). At each site, more than three samples were collected. Water, cyanobacterial mat (microbial film) and sediment samples were collected from hypersaline lakes, and water and microbial biofilm only from springs. The water samples were collected and placed in a plastic bottle (2 L). Surface sediment and microbial film samples were collected by hand. They were packed separately in airtight polyethylene bags. All samples were stored in an icebox until returned to the laboratory. The sediment and microbial film samples were dried in a circulating air oven at 105 °C, crushed and then sieved to a mesh size of 63 mesh.
Gamma-ray spectrometry
The natural and anthropogenic radioactivity activity concentrations were analysed using gamma spectrometry. The gamma spectrometer is based on a high-purity germanium (HpGe) detector. The 3 × 3NaI(Tl) detector, with a resolution of 1.90 keV and a peak/Compton ratio of 69.9:1 at the 1.33-MeV gamma-ray line of 60Co, has a relative efficiency of about 50%. It is coupled to conventional electronics connected to a multichannel analyser card (MCA) installed in a PC. The detector is shielded by a 10-cm thick lead liner and has a 2-mm copper foil lining inside. The MAESTRO software programme from ORTEC was used for the acquisition of the data and the analysis of the spectra. The spectrometer energy calibration was performed using point sources of 241Am (59.6 keV), 137Cs (661.6 keV) and 60Co (1172 and 1332.3 keV). The calibration of the efficiency of the spectrometer was carried out using three well-known reference materials, namely RGU-1, RGTh-1 and RGK-1 (IAEA 1987; Anjoset al. 2005; El-Aassy et al. 2012). The Marinelli beakers were tightly sealed for at least 4 weeks. This was to ensure that a state of secular equilibrium between radium and its daughters was reached. The time accumulation of the spectra was at least 48 h, depending on the activity of the samples. The activity of 226Ra (238U series) was measured using the 186.1-keV gamma-ray energy transition after subtracting the 185.7 keV of 235U, the 295.2 and 351.9 keV of 214Pb and the 609.3 and 1120.3 keV of 214Bi. The gamma energies of 338.4 keV and 911.2 keV for 228Ac, 583 keV and 2614.4 keV for 208Tl and 727.3 keV for 212Bi were taken to represent the concentrations of the 232Th series. 40K was measured directly from the 1460.8-keV gamma transition (Chieco et al. 1990). In addition, 137Cs was measured directly from 661.7 keV. The activity concentration of the radionuclides was calculated according to the formula of EL Afifi et al. (2006). Quality control (QC) is carried out to ensure that the analysis is accurate and to assess the validity and reliability of the data. The precision and accuracy of the analysis were estimated by measuring the radionuclides in the certified reference materials (e.g. IAEA-443, IAEA-446, IAEA-410 and IAEA-312). The measured activity concentrations, with mean deviations and errors not exceeding 3%, were very close to the reported values of the certified materials. The precision of the gamma spectrometer system is obtained by the lowest limits of detection (LLDs), which were determined according to USDOE (1992) and Abid Imtia et al. (2005). The lowest detection limits (LLDs) are 9.35, 0.45, 1.34 and 0.2 Bq kg−1 for 40K, 226Ra, 232Th and 137Cs, respectively, for the sediment. Otherwise, the LLD values for water are 0.84, 0.1, 0.1 and 0.02 Bq l−1 for 40K, 226Ra, 232Th and 137Cs, respectively.
Radiation hazard indices and potential health of the radionuclides
It is logical to use various known health risk indices to make a confident statement about human health and the environment. The radiation hazard indices are the absorbed dose rate in air (D), the annual effective dose rate (AED), the radium equivalent activity (Raeq), the external hazard index (Hex) and the representative level index (Iγ), calculated according to the equations given in Table 1.
The health effects sub-committee recommends that the risks associated with all radium isotopes should be combined to reflect the total risk. The cancer risk associated with ingesting radium isotopes was estimated via EPA 1999; Abdellah and Diab 2012.
where CR is lifetime cancer risk corresponding to MCL (unit less), MCL is the maximum contaminant level (Bq l−1), RC is the mortality risk coefficient for 226Ra (7.17 × 10–9 Bq l−1) and 228Ra (2.0 × 10–8 Bq l−1) and TWI is total water intake (2 L d−1 × 365.4 d year−1 × 70 years).
The effective radiation doses (DRW) resulting from the ingestion of these waters were assessed according to the USEPA (1999) in order to calculate potential health hazards.
where Aw is the activity concentration of radionuclides (Bq l−1), IRw is the water intake for a human being for 1 year and IDF is the effective dose in mSv Bq−1. For infants, children and adults, doses were calculated using a consumption rate of 150, 350 and 730 L yr−1, respectively. The conversion factors for infants, children and adults are given in Table 2.
Water-to-biota transfer factor (the bioaccumulation factor, BF)
The transfer factor (bioaccumulation factor, BF) was calculated from the concentrations of the radioactive or natural isotope in both water and biota (microbial films) samples to determine the rate of transfer of natural radioisotopes from water to biota in the study area. The bioaccumulation factor is expressed as the concentration of biotic tissue relative to the concentration of water at equilibrium (UNSCEAR 1977).
where Cbiota is radionuclide activity concentration of biota per kg fresh weight, and Cwater is radionuclide activity concentration of water per L.
Results and discussion
Environmental conditions
The mean temperature values of Siwa lakes ranged between 19.1 and 19.5 °C, and the mean TDS values varied from 178.47 to 198.61 g/l. According to Hassan and Ismail (2018), the limestone, dolomite and evaporite deposits of the Siwa Oasis have fractured and mixed with the trapped ancient seawater, resulting in the high salinity levels. In addition, there are extensive agricultural activities with high evaporation rates and high temperatures that increase TDS levels. Compared to Siwa lakes, spring’s water samples are characterised by high water temperature (31.2 °C in spring (1) to 53.2 °C in spring (3)) and very low TDS (0.8 g/l in spring (2) to 1.4 g/l in spring (3)). The studied water is rather alkaline side for lakes and springs. All water samples fall within the recommended range of 6.5–8.5 for irrigation, according to the USDA (2011).
Radioactivity measurement
Water
The activity concentrations of 226Ra, 232Th, 40K and 137Cs in water samples are from hypersaline lakes and freshwater springs. The activity concentrations of 226Ra and 232Th in water samples did not differ significantly between hypersaline lakes and freshwater springs (Table 3). The 226Ra activity concentration of hypersaline lakes ranged from 0.5 ± 0.05 Bq l−1 in Lake Siwa to 4.10 ± 0.49 Bq l−1 in Lake Zeitoun. For the springs, it ranged from 2.01 ± 0.19 Bq l−1 in spring (2) to 3.59 ± 0.40 Bq l−1 in spring (1). The high 226Ra concentrations in water samples indicate that the area of the aquifer matrix from which the water originates is enriched in 238U activity concentrations; 226Ra activities in groundwater are mainly controlled by the primary 238U content of the aquifer system (Lauria et al. 2004). Whereas; radium in groundwater can be derived from a number of sources, including (1) the decay of uranium or thorium parent nuclei dissolved in solution, (2) decay from formation minerals, (3) alpha-recoil release of radium from the decay of parent nuclei in the aquifer, (4) radium adsorbed on the surface layer, clays and oxides and (5) co-precipitation with the secondary minerals (Porcelli and Swarzenski 2003). In addition, the high 226Ra activity in hypersaline lakes could be the result of water runoff from local agriculture containing phosphate fertilisers. Hussein and Ahmed (1997) report an increase in 226Ra concentrations in the lakes as a result of the use of phosphogypsum and superphosphate fertilisers in agriculture. Although the mean activity concentration of 226Ra exceeded the internationally permissible value (1 Bq l−1) in the springs and hypersaline lakes, except for Lake Siwa, it was much lower than the value found by Allam (2019) and lower than the mean value found by Abd El-Mageed et al. (2013) in the hot springs and groundwater in Yemen. The elevated radium activity concentration observed in this study is consistent with previously published radium data for groundwater from the Nubian aquifer in the eastern desert of Egypt (Sherif & Sturchio 2018), the Nubian aquifer in the Western Desert of Egypt (Sherif & Sturchio 2021), the Sinai Peninsula in Egypt (Sherif et al. 2018) and the Disi aquifer in Jordan (Vengosh et al. 2009). The distribution and behaviour of the Ra isotopes in the groundwater is determined by the coupled effects on various geochemical processes (Sherif & Sturchio 2021). Compared to other sandstone aquifers worldwide, radium activity in ancient groundwaters in the Middle East and northeastern Africa is generally much higher than in other sandstone aquifers (Sherif & Sturchio 2021).
The levels of 232Th concentration are low in most samples, with the exception of spring (1) and Lake Siwa. The activity concentrations of 232Th ranged from 0.40 ± 0.05 Bq l−1 in spring (3) to 1.20 ± 0.09 Bql−1 in Lake Siwa. The mean activity concentrations of 232Th in the hypersaline lakes and springs were lower than the international allowable value (1 Bq l−1). These values were lower than the upper limits found by Allam (2019) in the Siwa region and comparable to those found by Mathuthu et al. (2021) in Namibian groundwater. Furthermore, the 232Th concentrations in freshwater wells in this study were much lower than the levels found by Yehia et al. (2017) in thirty wells and springs in the Western Desert, Egypt. 232Th is virtually immobile in groundwater, has not migrated elsewhere and is strongly adsorbed to iron hydroxides (Murphy et al. 1999). The lower values of the 232Th/226Ra ratio (0.13–0.33) in all samples, except those in Lake Siwa (1.14–1.83), indicate that radium in the Nubian sandstone aquifer is derived exclusively from interactions with rocks in the U decay series. In the same context, the activity concentrations of 40K are higher than the worldwide value in the hypersaline lakes and spring (1), as shown in Fig. 2. The activity concentrations of 40K ranged from 2.10 ± 0.13 Bql−1 at the spring (2) to 71.1 ± 1.90 Bql−1 at Lake Aghormi. The higher 40K activity observed in water samples from hypersaline lakes (Zeitoun, Aghormi and Siwa) was generally explained by the use of potassium fertiliser in agricultural activities. The 40K activity concentration in the springs ranged from 2.10 ± 0.13 to 6.40 ± 0.4 Bql−1, which was significantly lower than the values reported by Adetunji et al (2018) in Nigeria and Allam (2019) in Siwa area for underground water.
The activity concentrations of 137Cs ranged from 0.23 ± 0.08 Bq l−1 at spring (2) to 0.70 ± 0.05 Bq l−1 in Zeitoun Lake. The activity concentrations of 137Cs are lower than the worldwide range (WHO 2003; IAEA 1996) but were slightly higher than the values conducted by Zgorelec et al. (2021) in the acidic soil of northwestern Croatia. The fallout radionuclide 137Cs was detected in all the water samples (hypersaline and springs), which could be due to 137Cs falling from the atmosphere to the earth’s surface, where it could enter the groundwater circulation system. The advection and dispersion model was used to try to understand the mechanism of migration of Cs-137 into groundwater. It was assumed that the pore water in the uppermost 0.25 m of the soil was equilibrated with the Cs-137 fallout at the ground surface (GEO-SLOPE 1950), and the dissolved 137Cs migrated in the soil and reached aquifers at different depths (Shizuma et al. 2018). Furthermore, the vertical migration of 137Cs in aquifers is relatively weak and depends on the contaminant levels (fallout) and their forms (particles or dust) (Ashraf et al. 2014). As a result, there are small amounts of contamination distributed through the groundwater circulation system.
Sediment
The mean value of activity concentration of 226Ra, 232Th, 40K and 137Cs in sediment samples was found to be 24.5 ± 2.01, 11.52 ± 1.09, 201.33 ± 2.7 and 1.9 ± 0.11 Bq kg−1 (Table 3), respectively. Figure 3 shows the spatial distribution of activity concentrations for 226Ra, 232Th, 40K and 137Cs in sediment sample of hypersaline lakes that indicate levels below the permissible level of worldwide (UNSCEAR 2000). The results show that the order 137Cs < 232Th < 226Ra < 40K is the mean activity concentration for all sampling sites. The 232Th concentration is significantly lower than that of 226Ra and 40K. The elevated activity concentration of 226Ra in Lake Zeitoun could be due to the discharge of agricultural drainage water containing chemical fertilisers. It is assumed that the original materials making up the various geological formations of the area under study are the cause of the variance and frequency of radionuclide concentrations. These results suggest that atmospheric deposition in this area could be an explanation for the discovery of the 137Cs radionuclide in hypersaline lakes (Zeitoun, Aghormi and Siwa). The 137Cs radionuclide forms associations with mud minerals in the upper soil layer (Bourcier et al. 2011), and clay minerals can absorb caesium (Fan et al. 2014). In addition, most caesium constituents have a chemical affinity comparable to that of potassium (K) and are soluble in water (UNSCEAR 1982). Timofeeva-Resovskaya (1963) classified 137Cs as a pedotope, which means that it belongs to the group of elements deposited by bottom sediments in 95–100% of cases. Darwish et al. (2013) found that the mean activity concentration of 226Ra and 232Th of sediment in the saline Qarun Lake was consistent with this study and lower than the recommended value of soil (UNSCEAR 2000). Otherwise, Lui et al. (2021) reported high activity concentrations of 226Ra, 232Th and 40K in the bottom sediments of the Nansha Sea and South China Sea that were 14.6–38.51, 15.81–49.21 and 149.7–569.35 Bq kg−1, respectively.
Microbial films
The mean activity concentrations of 226Ra, 232Th, 40K and 137Cs were 10.73 ± 0.83, 7.73 ± 0.63, 316.17 ± 4.03 and 1.23 ± 0.1 Bq kg−1, respectively, in microbial films from hypersaline lakes (Table 3 and Fig. 4). In contrast, they were 215.93 ± 5.3, 130.17 ± 2.13, 305.20 ± 3.57 and 2.17 ± 0.23 Bq kg−1 in microbial films from hot springs, respectively. In the samples from the springs, the microbial films were 100 times more radioactive than the surrounding groundwater. The cyanobacterial community of the Siwa hot springs could serve as a biosorbent for radionuclides. These results indicate that 226Ra and 232Th are present at high concentrations in the microbial film samples from springs. This may be due to the fact that radium, a substance that readily penetrates plants due to its barrierless nature (Titaeva 2000), is also a unique biologically essential element (Vanov 1994). Furthermore, this suggests that 226Ra can adsorb to the capsule surfaces of cyanobacteria, cocci and bacilli in microbial mats, leading to the formation of extracellular polymer capsules and slime layers around the cells that protect against radionuclides and other metals in hot springs. It is conceivable that the ability of a particular bacterium to immobilise heavy metals could be used to mitigate the terrible effects of radioactive pollution (Tazaki 2009). Based on their similar chemical properties, it could be predicted that the two radionuclides 226Ra and 232Th behave similarly during the processes of precipitation and mineral formation (Lazareva et al. 2011). Furthermore, 40K concentrations varied within a narrow range in microbial film samples from hypersaline lakes, whereas they were widely separated in spring samples with the highest activity concentration in the spring (2). 137Cs concentrations were higher in microbial films of freshwater springs than from hypersaline lakes. In the same context, Lee et al. (2019) found that the uptake efficiency of 137Cs by Haematococcus pluvialis and Chlorella vulgaris was 95% and 90%, respectively. The movement and accumulation of Cs-137 in the air depends on the radionuclide particle size, climatic conditions and earth science. Bioaccumulation of radiocaesium in higher organisms is significantly influenced by several factors (e.g. pH, K+ and organic matter) (Avery 1995). Caesium is known to be transported into cells as a potassium analogue; consequently, the presence of potassium in the media interferes with 137Cs absorption (Whicker and Schultz 1982; Bystrzejewska-Piotrowska and Urban 2003). It has been shown to be taken up by plant and algal cells via potassium transport channels due to chemical similarities (Lee et al. 2019).
Lee et al. 2019 also showed that each microalgal species can selectively remove different elements; which explains why the extremophilic microbial film of Siwa Oasis, composed of different groups and many species, can remove different radionuclides from the surrounding media, which is more difficult than the remediation of a single form of contaminant, as reported by Thakare et al. (2021). Microbial films, especially those from freshwater sources, appear to be good accumulators of various radionuclides, which are several times higher in freshwater microbial films than in the surrounding media. In the global search by Shin-ya et al. (2014), the highly active strains for radionuclide removal were all freshwater strains. However, none of the marine strains could show high radionuclide removal efficiency because competing elements in seawater such as K and Ca would reduce the ability of cells to absorb or accumulate radionuclides (Shin-ya et al. 2014). The high content of metals in seawater results in lower accumulation of radionuclides by marine biota (Chebotina and Kulikov 1998) due to competition of salts with radionuclides for adsorption sites (Strand 1998).
The results showed that the distribution of 226Ra, 232Th, 40K and 137Cs in water, sediment and microbial films was not consistent. The radioactivity varied significantly depending on the different sources and the geography of the region. The findings suggest that water–rock interaction in sandstone aquifers can potentially mobilise radionuclides from the U and Th series, which may explain the higher levels of 226Ra and 232Th in the springs water and microbial film samples. Furthermore, freshwater microbial films indicate that they are good accumulators of different radionuclides. The distribution of radionuclides 226Ra, 232Th, 40K and 137Cs in hypersaline lakes also showed the influence of agriculture in this area. Using a global comparison, the activity concentration of radionuclides in groundwater, microbial films and sediment of Siwa Oasis is compared with the studies across different countries (Table 4). The mean activity concentration of 226Ra and 232Th of groundwater in this study agreed with the Marsa Alam-Shalateen area, Egypt (Arafat et al. 2017), and Siwa Oasis, Egypt (Saleh et al. 2016), and lower than Anarak-Khour a desertic area, Iran (Ehsanpour et al. 2014), and Al-Hurrah city in Najaf, Iraq (Alaboodi et al. 2020), and the activity concentration of 226Ra series was higher than the values obtained in Phra Nakhon Si Ayutthaya Province, Thailand (Kodcharin et al. 2018) and Jordan (Alomari et al. 2019). Also, the activity concentration of 40K was lower than Al-Hurrah city in Najaf, Iraq (Alaboodi et al. 2020), and higher than in Phra Nakhon Si Ayutthaya Province, Thailand (Kodcharin et al. 2018), Anarak-Khour a desertic area, Iran (Ehsanpour et al. 2014), Marsa Alam-Shalateen area, Egypt (Arafat et al. 2017), and Siwa Oasis, Egypt (Saleh et al. 2016). The activities of 226Ra and 232Th of microbial films in Siwa were higher than Gulf of Suez, Egypt (Diab et al. 2019), plants in Siwa Oasis, Egypt (El Begawy et al. 2019), and moss in the Marmara region, Turkey (Belivermis and Çotuk 2010). Furthermore, the activity concentration of 40K was lower than Gulf of Suez, Egypt (Diab et al. 2019), plant in Siwa Oasis, Egypt (El Begawy et al. 2019), and Iskenderun Bay, Turkey (Varinlioğlu et al. 1997), but higher than moss in Marmara region, Turkey (Belivermis and Çotuk 2010). The activity concentrations of 226Ra, 232Th and 40K in the sediment were lower than in Lake Nasser (Imam et al. 2020).
Environmental hazard indices and risk assessment
The radiological hazards indices are radium equivalent activity (Raeq), absorbed dose rates (D), annual effective dose (AED), external hazard (Hex) and the representative gamma index (Iγ) in microbial films, and sediment was determined and listed in Table 5. The mean radium equivalent activity (Raeq), absorbed dose rates (D), annual effective dose (AEDs), external hazard (Hex) and the representative gamma index (Iγ) are 54.84 ± 3.76 Bq kg-1, 26.68 ± 1.7 nGyh-1, 0.03 ± 0.002 mSvy-1, 0.15 ± 0.01 and 0.41 ± 0.03, respectively. These radiation indices of sediment are less than the reference maximum levels provided by the UNSCEAR (2000). This indicates that although the hypersaline lakes’ sediment being contaminated with chemical fertiliser of the agriculture activity in this area, the results of the measurements are still within acceptable ranges. Similarly, the findings of environmental hazard of the microbial films showed that the Raeq values varied from 41.19 to 602.6 Bq kg−1 with mean value of 233 ± 5.3 Bqkg−1, and the mean absorbed dose rate (D) and annual effective dose (AEDs) outdoors were 107.45 ± 2.39 nGyh−1 and 0.132 ± 0.003 mSvy−1, respectively, with the highest value in spring (2). The calculated value of the external hazard indices of the microbial films due to natural radioactivity 226Ra, 232Th and 40K was lower than unity at all sites except at springs (2) and (3). In the same context, the representative level index (Iγ) ranged from 0.35 to 4.24, with a mean value of 1.65 that higher than the recommended value (< 1). Radium equivalent activities, annual outdoor effective doses (AEDs) and external hazard index Hex in microbial films were lower than the UNSCEAR (2000) permissible levels.
Otherwise, the absorbed dose rate (D) and the representative level index (Iγ) in microbial films were higher than the recommended values of the UNSCEAR (2000) (18–93) nGyh−1 for (D) and less than unity for (Iγ), respectively. This could be due to the fact that the calculated absorbed dose of the microbial films is used as an equivalent for the dose absorbed by living tissue. According to Lloyd and Lovley (2001), Shukla et al. (2017), Lopez-Fernandez et al. (2020) and Pande et al. (2022), the mechanisms of microbial tolerance to radiotoxicity from radionuclides are primarily realised through various forms of interaction between microbes and radionuclides, such as, e.g. biomineralisation, biosorption, bioaccumulation and biotransformation. In the technique of biosorption, radioactive cations are taken up by bacteria through the cell scaffold and then adsorbed onto binding sites such as amine, carboxyl, hydroxyl and other groups in the cell walls. Adsorption has been found to help many types of organisms reduce the harmful effects of a variety of radionuclides (Shukla et al 2017; Lopez-Fernandez et al 2020).
Under the above circumstances, the effective dose was estimated for different age groups, infants, children and adults, considering the intake of 226Ra, 232Th and 40K in Siwa groundwater (see Table 5 and Fig. 5). The mean annual effective doses (DRW) of radiation during water consumption for all wells/springs in this study area for infants, children and adults were 0.49, 0.91 and 0.73 mSv/year, respectively. The annual effective dose (DRW) for infants, children and adults was below the minimum risk level (MRL) of 1 mSv/year as reported by ICRP 2000. But also, it was about two, five and seven times higher, for infants, children and adults, respectively, than reported in the WHO 2003. The high values of effective dose in the springs samples could be due to the high radium concentration. These results of the effective dose are consistent with those obtained by Saleh et al. (2016) in groundwater of the Siwa Oasis, lower than those obtained by El-Mageed et al. (2013) from ground and hot spring water in Yemen and higher than those obtained by Faanu et al. (2016) from underground water in the open areas for gold mining and processing in Ghana. The cancer risk associated with lifetime (70 years) intake of radium isotopes in water samples (226Ra and 232Th) ranged from (135.6 ± 17.2) × 10–5 to (244 ± 29.3) × 10–5 with an average value of (178.1 ± 22.2) × 10–5, which is slightly higher than the USEPA (2000) range (1 × 10–4–1 × 10–6). Natural springs, shallow and deep wells and numerous springs are distributed across Siwa Oasis. These springs are used for drinking, irrigation and medical tourism (medical treatment).
We selected three springs for our study: Spring (1) is used for irrigation, spring (2) is utilised for medical tourism (spa) and spring (3) is used for drinking, irrigation and feeding a mineral water company. The mean values of the estimated effective dose from ingesting 226Ra, 232Th and 40K and the excess lifetime cancer risk in groundwater from this study were higher than the globally acceptable value. This is indicated that groundwater has to be treated to reduce the levels of radioactive contaminants before being utilised for drinking and other domestic purposes. Although there are no immediate public health effects at this time, it is likely that there will be long-term, accumulative health effects in this range. This suggests the need for environmental investigations and monitoring of wells and springs for the distribution of minerals and radionuclides in this region.
Bioaccumulation factor
The bioaccumulative properties of microbial films toward 226Ra, 232Th, 40K and 137Cs contamination may be very effective for potential application in monitoring and assessment of extreme habitats, but only in a short term, since the corresponding turnover rates are quite short. The other rationale for using microbial films for radionuclide monitoring purposes is their ease of sampling, continuous growth processes, rapid response to the contamination and the consideration as a unique biological system that can grow in such extreme habitats. Microbial films are good indicators and accumulators of radionuclides in Siwa extreme habitats, considering the very short lifespan of microbial films (from days to a few weeks) compared to the long lifespan of marine macroalgae. Moreover, these results may indicate the further ability of these microbial films to bioremediate radionuclides contaminated.
Our data have been described as the activity concentration for the dry weight, multiplied by 0.16 (Kulikov and Molchanova 1975), to be expressed as the fresh weight. In general, the highest transfer factor was observed for 232Th, followed by 40K, and 226Ra (Table 6). The transfer factors of 226Ra, 232Th, 40K and 137Cs calculated for the microbial film samples collected from Siwa Lakes were 0.87, 1.59, 2.68 and 0.5, respectively. For the freshwater springs, the transfer factors of 226Ra, 232Th, 40K and 137Cs calculated for the microbial film were 13.04, 40.77, 20.69 and 1.29, respectively. It is obvious that the highest transfer factor in hypersaline lakes was 40K, while in springs, it was 232Th. It is normal that the transfer factor of 40K is high in lakes, while potassium is more abundant in hypersaline lakes as it is an essential element in soils and plants and is used as phosphate fertiliser. Otherwise, the transfer factor of 232Th is high due to the low solubility of thorium in water, which explains the complete transfer of thorium from water into biota (microbial films). Similarly, the transfer factor of 226Ra is lower than that of 232Th, which is due to the fact that part of the radium is lost as a dissolved and precipitated substance in groundwater that some of the radium is loss as dissolved and precipitates in groundwater. However, the Siwa Oasis microbial systems can sequester 226Ra (238U series), 232Th series and 40K at concentrations higher than those found in the surrounding water, making algae superior to other aquatic biotas for radionuclide monitoring, especially in systems where such biotic elements are lacking. Extremophilic microbial films can remove different radionuclides from surrounding media because each group/species selectively removes specific elements.
The accumulation of radionuclides in biota depends on the biological availability of the absorbed radionuclides, differences in biota size and lifespan, food chain variations and vertical distribution in the water column (Mito et al. 1999). Even though the life span of microbial films is short (days or weeks), they tend to accumulate high concentrations of radioactive nuclides in comparison with sediments. Microbial films are a consortium of cyanobacteria and bacteria embedded in gelatinous polysaccharide secretions. Such consortium has been reported to efficiently adsorb heavy metals (Ali et al. 2019).
The efficiency of radionuclide removal is influenced by the amount of viscous polysaccharide molecules covering the cell surface (Hill et al., 1997; Tamaru et al., 2005); these extracellular polysaccharides are capable of adsorbing U (Kalina et al., 2005). Most plants, algae and bacteria have negatively charged polysaccharides and carbohydrates on the surface of their cell walls (Myers et al. 1975; Lobban and Wynne 1981). Zn, Cu, Al and U are attracted to the negatively charged groups (Marques et al. 1990; Barker et al. 1998). Many additional side groups, such as amino, carboxyl, hydroxyl and sulphide, found in the polysaccharide backbone of cell walls, provide an additional net negative charge to the cell. However, certain groups have a positive charge and can adsorb complex anionic metal species. The number of cations and anions complexed is determined by the mixture of these groups on the cell surface. For a representative phytoplankter, the possible number of high-affinity surface binding sites is estimated to be about 107–108 per cell (Morel and Hudson 1985). On the bacterium Shewanella putrefaciens, the number of ligand sites for U(VI) was determined to be 2.0 × 1019 carboxyl sites, 5.5 × 1018 phosphoryl sites and 2.3 × 1019 amine sites per gram of bacteria (Haas et al. 2001). Mycobacterium smegmatis cell walls could probably adsorb about 100-mg U per gram of dry cell weight, according to Andres et al. (1994).
Conclusion
Groundwater is the only source of water that sustains life in the Siwa Oasis. The increasing use of groundwater for drinking and agricultural purposes requires careful assessment of radioactive substances and their radiological risks to public and environmental health, and the transfer of radionuclides to biota must be considered in the initial stages of an environmental impact assessment. The results showed that the elements of the radionuclides in different environmental samples (water, sediment and microbial films) vary considerably, depending on the different sources and on the geography of the region. The mean activity concentrations of 232Th and 137Cs in the groundwater were in agreement with the recommended reference values, while the concentrations of 226Ra and 40K were higher than the reference value. Furthermore, the mean concentrations of radionuclides in microbial films and sediment were within the global value, except for 226Ra and 232Th in microbial film samples. Furthermore, the microbial films from the freshwater suggest that they are good accumulators of various radionuclides. The influence of agriculture in this area was also shown by the distribution of the radionuclides 226Ra, 232Th, 40K and 137Cs in hypersaline lakes. Regarding radiation hazards, the radium equivalent activity, external hazard and annual effective dose of the microbial films and sediment samples were below the permissible limits. Otherwise, the absorbed dose rates (D) and the representative level index (Iγ) were higher than the recommended value, which could represent the ability of the microbial films to absorb the absorbed dose into living tissue. However, the annual effective dose to infants, children and adults was below the ICRP 2000, MRLs of 1 mSv/year. The annual cancer risk from radionuclides in the Siwa groundwater varied within a narrow range with an average value of 178.1 × 10–5, unacceptable when compared with the reference value.
The bioaccumulation factors of the radionuclide elements calculated for the microbial film samples collected from the freshwater springs are higher than the transfer factors of the radionuclide elements calculated from the hypersaline lakes. The microbial films studied can absorb 226Ra, 40K and 137Cs from their living media. Furthermore, these microbial films have a high capacity for the uptake of 232Th, in spite of the low solubility in water. The bioaccumulation efficiency of microbial films in the extreme habitats of Siwa Oasis toward radionuclides is very useful for potential applications in monitoring, assessing and remediating these habitats. This study has shown that the microbial films in the Siwa Oasis are able to tolerate not only high salinity and high temperature, but also high levels of radioactivity. The rationale for using these microbial films is simple: They are abundant, easily accessible and grow continuously. Most importantly, microbial films are the only ones living in these extreme habitats. For further bioindicators and bioremediation purposes, we need to gather basic information on the physiology and growth requirements of microbial films and their bioaccumulation properties toward radionuclides or heavy metals.
Data availability
The datasets and materials used during the current study are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article.
References
Abd El-Mageed AI, Abd El-Hadi E, Abd El-Bast A, Shaban H, Imran IS (2013) Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination 321:28–31
Abdellah WM, Diab HM (2012) Determination of natural radioactivity in drinking water and consequent dose to public. Nat Sci 10(1):137–142
Adetunji A, Olorunfemi AO, Abe O, Adesiyan TA (2018) Anomalous concentrations of radionuclides in the groundwater of Ede area, southwestern Nigeria a direct impact of geology. Environ Earth Sci 77:618
Alaboodi AS, Kadhim NA, Abojassim AA, Baqir Hassan A (2020) Radiological hazards due to natural radioactivity and radon concentrations in water samples at Al-Hurrah city Iraq. Int J Radiat Res 18(1):1–11
Ali MHH, AbdElkarim MS, Haroun SA, Attwa KM (2019) Bioremediation of Fe, Zn and Cd ions from aqueous solution using died cells of cyanobacterial mats from extreme habitat, Siwa Oasis Egypt. Egypt J Aquatic Biol Fish 22(5):511–522
Allam KA (2019) Radiological risk estimation of drinking and irrigation water from Siwa Oasis, Western Desert. Egypt J Radiat Nucl Appl 4(3):193–199
Al-Masri MS (2003) Marine environment studies on the Syrian Coast: biomonitors. In: Proceeding of CIESMWorkshop–Mediterranean MusselWatch, 18–20 April 2002, Marseille, France, pp. 3–6.
Alomari AH, Saleh MA, Hashim S, Alsayaheen A, Abdeldin I (2019) Activity concentrations of 226Ra, 228Ra, 222Rn and their health impact in the groundwater of Jordan. J Radioanal Nucl Chem 322(2):305–318. https://doi.org/10.1007/s10967-019-06686-4
Al-Qahtani KM, Ali MH, Abdelkarim MS, Al-Afify AD (2021) Efficiency of extremophilic microbial mats for removing Pb(II), Cu(II), and Ni(II) ions from aqueous solutions. Environ Sci Pollut Res 28:53365–53378
Andres Y, MacCordick HJ, Hubert JC (1994) Binding sites of sorbed uranyl ion in the cell wall of mycobacterial biomass. FEMS Microbiol Lett 115:27–32
Anjos RM, Veiga R, Soares T, Santos AMA, Aguiar JG, Frascá MH, Brage JAP, Uzeda D, Mangia L, Facure A, Mosquera B, Carvalho C, Gomes PRS (2005) Natural radionuclide distribution in the Brazilian commercial granites. J Radiat Measure 39:245–253
Arafat AA, Salama MHM, El-Sayed SA, Elfeel AA (2017) Distribution of natural radionuclides and assessment of the associated hazards in the environment of Marsa Alam-Shalateen area, Red Sea coast. Egypt J Radiat Res Appl Sci 10(3):219–232. https://doi.org/10.1016/j.jrras.2016.11.006
Ashraf MA, Akib S, Maah MJ, Yusoff I, Balkhair KS (2014) Cesium-137: radio-chemistry, fate, and transport, remediation, and future concerns. Crit Rev Environ Sci Technol 44(15):1740–1793
Avery SV (1995) Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J Ind Microbiol 14:76–84. https://doi.org/10.1007/BF01569888
Baba A, Tayfur G (2011) Groundwater contamination and its effect on health in Turkey. Environ Monit Assess 183:77–94
Barker WW, Haas JR, Suzuki Y, Banfield JF (1998) U-Phosphate biomineralization as a mechanism of U fixation by lichen, Geological Society of America, Annual Meeting.
Belivermiş M, Çotuk Y (2010) Radioactivity measurements in moss (Hypnum cupressiforme) and lichen (Cladonia rangiformis) samples collected from Marmara region of Turkey. J Environ Radioact 101(11):945–951. https://doi.org/10.1016/j.jenvrad.2010.06.012
Bourcier L, Masson O, Laj P, Pichon JM, Paulat P, Freney E, Sellegri K (2011) Comparative trends and seasonal variation of 7Be, 210Pb and 137Cs at two altitude sites in the central part of France. J Environ Radioact 102(3):294–301. https://doi.org/10.1016/j.jenvrad.2010.12.005
Brown J, Børretzen P, Dowdall M, Sazykina T, Kryshev I (2004) The derivation of transfer parameters in the assessment of radiological impacts on Arctic marine biota. Arctic 57(3):279–289
Bystrzejewska-Piotrowska G, Urban PL (2003) Accumulation of cesium in leaves of Lepidium sativum and its influence on photosynthesis and transpiration. Acta Biol Cracov Bot 45:131–137
Canu IG, Laurent O, Pires N et al (2011) Commentary health effects of naturally radioactive water ingestion: the need for enhanced studies. Environ Health Perspect 19:1676–1680
Chad-Umoren YE, Umoh IJ (2017) A gamma spectrometric analysis and radium equivalent activity index ofwater in ABAK, Nigeria: a base- line survey. Environ Earth Sci 7(5):19–26
Chebotina MY, Kulikov NV (1998) Ecological aspects of investigations of radionuclide migration in the continental basins. Ecologiya 4:282–290
Chieco NA, Bogen DC, Knutson EO (1990) Environmental Measurements Laboratory (EML) procedures manual.
Dahab KA (2004) Impact of the present groundwater exploitation system on the Nubia sandstone aquifer in Siwa Oasis, Western Desert, Egypt: 6th Intern. Conf. on Geochemistry, Alex.Univ., Egypt 15–16 Sept., 2004, 319–337
Darwish SM, El-Bahi SM, Sroor AT, Arhoma NF (2013) Natural Radioactivity Assessment and Radiological Hazards in Soils from Qarun Lake and Wadi El Rayan in Faiyum. Egypt Open J Soil Sci 3(7):289–296
Diab HM, Ramadan AB, Monged MHE, Shahin M (2019) Environmental assessment of radionuclides levels and some heavy metals pollution along Gulf of Suez. Egypt Environ Sci Poll Res 26(12):12346–12358. https://doi.org/10.1007/s11356-019-04610-7
Ebraheem AM, Garamoon HK, Riad S, Wycisk P, Seif El Nasr AM (2003) Numerical modeling of groundwater resource management options in the East Oweinat area, SW Egypt. Environ Geol 44:433–447. https://doi.org/10.1007/s00254-003-0778-1
Ehsanpour E, Abdi MR, Mostajaboddavati M, Bagheri H (2014) 226Ra, 232Th and 40K contents in water samples in part of central deserts in Iran and their potential radiological risk to human population. J Environ Health Sci Eng 12(1):1–7. https://doi.org/10.1186/2052-336X-12-80
El Afifi EM, Hilal MA, Khalifa SM, Aly HF (2006) Evaluation of U, Th, K and emanated radon in some NORM and TENORM samples. J Radiat Measur 41:627–633
El Begawy H, Salama MH, Ali HMH (2019) Evaluation of natural radioactivity levels and transfer factor of soil and plant, Siwa Oasis. Egypt Int J Environ Sci 2(1):46–60
El-Aassy IE, Nada AA, El Galy MM, El Feky MG, Abd El Maksoud TM, Talaat SM, Ibrahim EM (2012) Behavior and environmental impacts of radionuclides during the hydrometallurgy of calcareousand argillaceous rocks, southwestern Sinai. Egypt J Appl Rad Isot 70(6):1024–1033
Elkaffas SM, Massoud AM, El-Raey M, El-Wakeel H (2013) Spatial decision support system for water wells mining in Siwa Oasis. Int J Water Resour Arid Environ 2(3):158–163
El-Karim MS, Goher ME (2016) Heavy metal concentrations in cyanobacterial mats and underlying sediments in some Northern Western desert lakes of Egypt. J Fish Aquat Sci 11(2):163–173
El-Mageed AIA, El-Kamel AEH, Abbady AEB, Harb S, Saleh II (2013) Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination 321:28–31. https://doi.org/10.1016/j.desal.2011.11.022
El-Sayed SA, Allam KhA, Salama MH, El Begawy H (2017) Investigation of chemical and radiochemical fingerprints of water resources in Siwa Oasis, Western Desert. Egypt Arab J Nucl Sci Appl 50(1):158–178
Environmental Protection Agency (EPA) (1999) Final draft for the drinking water criteria document on radium. Washington, DC, Tr-1241- 85.
EPA, (2006) Consumer’s Guide to Radon Reduction, 402-K-06–094, December 2006.
Faanu A, Adukpo OK, Kansana C, Lordford T, Henry L, Kpeglo DO (2016) Assessment of naturally occurring radioactive materials on the public gold mining and processing at Newmont Golden Ridge Limited, Akyem Eastern Region of Ghana. Radiat Prot Environ 39(3):155–164. https://doi.org/10.4103/0972-0464.194962
Fan QH, Tanaka M, Tanaka K, Sakaguchi A, Takahashi Y (2014) An EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. Geochim Cosmochim Acta 135:49–65
Gad M, Dahab K, Ibrahim H (2018) Applying of a geochemical model on the Nubian sandstone aquifer in Siwa Oasis, Western Desert. Egypt Environ Earth Sci 77(11):1–14. https://doi.org/10.1007/s12665-018-7580-6
Garza D (2005) Common edible seaweeds in the Gulf of Alaska. Alaska Sea Grant Program, Fairbanks
Gazso LG (2001) The key microbial processes in the removal of toxic metals and radionuclides from the environment. Central Eur J Occup Environ Med. 7(3/4):178–185
Geo-Slope, I. (1950). Caesium-137 Transport in an Unconfined Aquifer, 1–9.www.geo-slope.com
Gindy AR, El-Askary MA (1969) Stratigraphy, structure, and origin of Siwa Depression, Western Desert of Egypt. Bull Am Assoc Pet Geol 53:603–625
Gossel W, Ebraheem AM, Wycisk P (2004) A very large scale GIS based groundwater flow model for the Nubian Sandstone aquifer in eastern Sahara Egypt, northern Sudan, and eastern Libya. J Hydrol 12:698–713
Haas JR, Dichristina TJ, Wade JR (2001) Thermodynamics of U(VI) sorption onto Shewanella putrefaciens. Chem Geol 180:33–54
Hassan S, Ismail E (2018) Detection and evaluation of ground- water in Siwa Oasis, Egypt using hydrogeochemical and remote sensing data analysis. Water Environ Res. https://doi.org/10.2175/106143017X15131012153004
Hussein, E. M. and Ahmed, H. A. H. (1997). Approach to the effect of phosphatic fertilizer and phosphogypsum upon the radioelement contents of some cultivated lands in the Egyptian nile delta." Egypt.
IAEA (1996) International Basic Safety Standards for Protection against Ionizing Radiation and for the Safety of Radiation Sources. Safety Series 15, Vienna.
Imam N, El-Sayed SM, Goher ME (2020) Risk assessments and spatial distributions of natural radioactivity and heavy metals in Nasser Lake. Egypt Environ Sci Poll Res 27:25475–25493. https://doi.org/10.1007/s11356-020-08918-7
Imtiaz MA, Begum A, Mollah AS, Zaman MA (2005) Measurements of radioactivity in books and calculations of resultant eye doses to readers. Health Phys 88(2):169–174
International Atomic Energy Agency (IAEA) (1987) Advisory Material for the IAEA Regulations for the Safe Transportof Radioactive Material (1985 Edition), Safety Series No. 37, 3rd edn. IAEA, Vienna
International Commission on Radiological Protection (ICRP), (2000).Protection of the the public in situations of prolonged radiation exposure. ICRP Publication 82, Pergamon Press, Oxford, United Kingdom.
Ivanov VV (1994) Environmental Geochemsitry. Nedra, Moscow ([in Russian])
Khater, A. 1998. Radiological study on the environmental behavior of some radionuclides in aquatic ecosystem”, Ph.D. thesis, Faculty of Science, Cairo University.
Khodashenas A, Roayaei E, Abtahi SM, Ardalani E (2012) Evaluation of naturally occurring radioactive materials (NORM) in the South Western oil wells of Iran. J Environ Radioact 109:71–75. https://doi.org/10.1016/j.jenvrad.2012.01.014
Kodcharin P, Youngchuay U, Chinwetkitvanich S (2018) Natural radioactivity in groundwater in phra Nakhon Si ayutthaya province. Appl Environ Res 40(3):11–18
Kulikov NV, Molchanova IV (1975) Continental Radioecology. Soil and Freshwater Ecosystems, Nauka, Moscow (in Russian).
Kurnaz A, KüçÜkömeroğlu B, Keser R, Okumusoglu TN, Korkmaz F, Karahan G, Çevik U (2007) Determination of radioactivity levels and hazards of soil and sediment samples in Frtna Valley (Rize, Turkey). Appl Radiat Isot 65:1281–1289
Lauria D, Almeida R, Sracek O (2004) Behavior of radium, thorium and uranium in groundwater near the Buena Lagoon in the coastal zone of the State of Rio de Janeiro. Brazil Environ Geol 47(1):11–19
Lazareva EV, Zhmodik SM, Melgunov MS, Petrova IV, Bryanskaya AV (2011) Redistribution of radionuclides between a microbial mat and a carbonate body at the Garga hot spring (Baikal Rift Zone). Dokl Earth Sci 439(2):1131–1137. https://doi.org/10.1134/S1028334X11080174
Lee K, Sang-Hyo L, Ju E, Seung-Yop L (2019) Biosorption of radioactive cesium from contaminated water by microalgae Haematococcus pluvialis and Chlorella vulgaris. J Environ Manage 233:83–88
Liu J, Anguo P, Shuang D, Min L, Guangshan L, Chao L (2021) Distribution of heavy metals and radionuclides in the sediments and their environmental impacts in Nansha Sea area. South China Sea Mar Poll Bull 166:112192
Lloyd JR, Lovley DR (2001) Microbial detoxification of metals and radionuclides. Curr Opin Biotech 12(3):248–253
Lobban CS, Wynne MJ (1981) The Biology of Seaweeds. Blackwell Scientific Publications, Oxford
Lopez-Fernandez M, Jroundi F, Ruiz-Fresneda MA, Merroun ML (2020) Microbial interaction with and tolerance of radionuclides: underlying mechanisms and biotechnological applications. Microb Biotechnol 14(3):810–828
Marques AM, Bonet R, Simon-Pujol MD, Fuste MC, Congregado F (1990) Removal of uranium by an exopolysaccharide from Pseudomonas sp. Appl Microbiol Biotechnol 34:429–431
Masoud A, Koike K (2006) Tectonic architecture through Landsat-7 ETM+/SRTMDEM-derived lineaments and relationship to the hydrogeologicsetting in Siwa region, NW Egypt. J Afr Earth Sc 45:467–477
Mathuthu M, Vera U, Vaino I (2021) Radiological safety of groundwater around a uranium mine in Namibia. Phys Chem Earth 122:102915
Mito K, Nishimura A, Yanagimoto T (1999) Ecology of groundfishes in the eastern Bering Sea, with emphasis on food habits. Dynamics of the Bering Sea. University of Alaska, Fairbanks, pp 537–580
Morel FMM, Hudson RJM (1985) The geobiological cycle of trace elements in aquatic systems: Redfield revisited. In: Stumm E (ed) Chemical Process in Lakes. Wiley, New York, pp 251–281
Murad A, Zhou XD, Yi P, Alshamsi D, Aldahan A, Hou XL, Yu ZB (2014) Natural radioactivity in groundwater from the south-eastern Arabian Peninsula and environmental implications. Environ Monit Assess 186:6157–6167
Murphy RJ, Lenhart JJ, Honeyman BD (1999) The sorption of thorium (IV) and uranium (VI) to hematite in the presence of natural organic matter. Coll Surf A 157:47–62
Myers VB, Iverson RL, Harriss RC (1975) The effect of salinity and dissolved organic matter on the surface charge characteristics of some euryhaline phytoplankton. J Exp Mar Biol Ecol 17:59–68
Nriagu J, Nam D-H, Ayanwola TA et al (2012) High levels of uranium in groundwater of Ulaanbaatar, Mongolia. Sci Total Environ 414:722–762. https://doi.org/10.1016/j.scitotenv.2011.11.037
Pande V, Pandey SC, Sati D, Bhatt P, Samant M (2022) Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front Microbiol 13:824084
Porcelli D, Swarzenski PW (2003) The behavior of U- and Th-seriesnuclides in groundwater. Rev Mineral Geochem 52:317–361
Saleh I, Elnaggar A, Ibrahim H (2016) Risk assessment of radionuclides in groundwater in siwa oasis Egypt. Int J Adv Res 4(11):1459–1466
Schubert M, Michelsen N, Rausch R (2011) Investigation and treatment of natural radioactivity in large-scale sandstone aquifer systems investigation and treatment of natural radioactivity in large-scale sandstone aquifer systems. Int J Water Res Arid Environ 1(1):25–32
Sherif MI, Sturchio NC (2018) Radionuclide geochemistry of groundwater in the Eastern Desert. Egypt Appl Geochem 93:69–80
Sherif MI, Sturchio NC (2021) Elevated radium levels in Nubian Aquifer groundwater of Northeastern Africa. Sci Rep 11:78. https://doi.org/10.1038/s41598-020-80160-0
Sherif MI, Lin J, Poghosyan A, Abouelmagd A, Sultan MI, Sturchio NC (2018) Geological and hydrogeochemical controls on radium isotopes in groundwater of the Sinai Peninsula Egypt. Sci Total Environ 613–614:877–885
Shin-ya F, Iwamoto K, Atsumi M, Yokoyama A, Nakayama T, Ishida K-I, Inouye I, Shiraiwa Y (2014) Global searches for microalgae and aquatic plants that can eliminate radioactive cesium, iodine and strontium from the radio-polluted aquatic environment: a bioremediation strategy. J Plant Res 127:79–89
Shizuma K, Fujikawa Y, Kurihara M, Sakurai Y (2018) Identification and temporal decrease of 137Cs and 134Cs in groundwater in Minami-Soma City following the accident at the Fukushima Dai-ichi nuclear power plant. Environ Pollut 234:1–8. https://doi.org/10.1016/j.envpol.2017.11.018. (Epub 2017 Nov 17 PMID: 29154205)
Shukla A, Parmar P, Saraf M (2017) Radiation, radionuclides and bacteria: an in-perspective review. J Environ Radio 180:27–35
Strand, P. (1998) Radioactivity, AMAP, Assessment Report: Arctic Pollution Issues, Oslo. P., 526–552.
Strezov A, Nonova T (2005) Radionuclide accumulation in green and brown macroalgae at the Bulgarian Black Sea coast. J Radioanal Nucl Chem 265(1):21–29
Tazaki K (2009) Observation of microbial mats in radioactive hot springs. Sci Rep Kanazawa Univ 53:25–37
Timofeeva-Resovskaya EA (1963) Distribution of radioisotopes in the main components of freshwater reservoirs. Tr Inst Biol Ural Fil AN SSSR 30:1–77 ((In Russian))
Titaeva,N. A. (2000). Nuclear Geochemistry (Moscow University Press, Moscow, 2000) [in Russian].
United Uations Scientific Committee on the effects of Atomic Radiation (UNSCEAR) (1977) Sources and Effects of Ionizing Radiation.
UNSCEAR. (1982). Ionizing radiation: sources and biological effects. UNSCEAR 1982 Report. US Scientific Committee on the Effects of Atomic Radiation. Available from https://www.unscear.org/unscear/en/ publications/1982.html.
UNSCEAR (2000) Ionizing Radiation:Sources and Effects on Ionizing Radiation. Report to the General Assembly, with scientific annexes, United Nations, New York.
USDA. (2011). Assessing water quality for human consump- tion, agriculture, and aquatic life uses tom pick. United States Department of Agriculture, National Agricultural Statistics Service.
USEPA, United States Environmental Protection Agency (1999) Final draft for the drinking water criteria document on radium. Washington, Dc, 1999 Tr-1241–85.
USEPA (2000) Quality Criteria for Water. EPA 440/5-86-001. 2001. Office of water regulations and standards. Washington DC., USA
Varinlioǧlu A, Küçükcezzar R, Köse A (1997) Radioecological measurements in the algae from Iskenderun Bay. Toxicol Environ Chem 64(1–4):75–79. https://doi.org/10.1080/02772249709358541
Vengosh A, Hirschfeld D, Vinson D, Dwyer G, Raanan H, Rimawi O, Al-Zoubi A, Akkawi E, Marie A, Haquin G, Zaarur S, Ganor J (2009) High naturally occurring radioactivity in fossil groundwater from the Middle East. Environ Sci Technol 43(6):1769–1775
Walencik A et al (2010) Natural radioactivity in underground waters. Pol J Environ Stud 19:461–465
Whicker FW, Schultz V (1982) Radioecology: Nuclear Energy and the Environment. CRC Press, Florida
World Health Organization (WHO) (2003) Guidelines for drinking water quality, vol.3-Chapter 9 draft, Geneva, Switzerland.
Yehia M, Baghdady A, Howari FM, Awad S, Gad A (2017) Natural radioactivity and groundwater quality assessment in the northern area of the Western Desert of Egypt. J Hydrol Reg Stud. https://doi.org/10.1016/j.ejrh.2017.06.002
Yuce G, Ugurluoglu D, Dilaver AT, Eser T, Sayin M, Donmez M, Ozcelik S, Aydin F (2009) The effects of lithology on water pollution: natural radioactivity and trace elements in water resources of Eskisehir region (Turkey). Water Air Soil Pollut 202:69–89
Zalewska T, Saniewski M (2011) Bioaccumulation of gamma emitting radionuclides in red algae from the Baltic Sea under laboratory conditions. Oceanologia 53(2):631–650. https://doi.org/10.5697/oc.53-2.631
Zgorelec Z, Marko S, Dinko B, Ivana S, Milan M, Aleksandra P, Branko P (2021) Effects of fertilisation on radionuclide uptake by maize from an acidic soil in northwestern Croatia. Soil Tillage Res 212:105030
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was supported by National Institute of Oceanography and Fisheries.
Author information
Authors and Affiliations
Contributions
Project administration, sampling and writing, review and editing were performed by MSA. Conceptualisation, validation, material preparation, data analysis and writing—original draft, review and editing were performed by NI.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial responsibility: Jing Chen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelkarim, M.S., Imam, N. Radiation hazards and extremophiles bioaccumulation of radionuclides from hypersaline lakes and hot springs. Int. J. Environ. Sci. Technol. 21, 3021–3036 (2024). https://doi.org/10.1007/s13762-023-05154-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05154-7