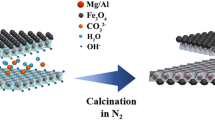
Abstract
Fuchsin acid (FA) and other organic dye contaminants have a great harm to the environment and organisms. It is of importance to develop suitable materials with simple preparation process, low cost and excellent performance, for the purification of polluted water. Layered double hydroxides (LDH), as a kind of efficient anion adsorption materials, can be reused simply, but with poor solid–liquid separation efficiency. Herein, the magnetized modified CaAl-LDH (designated as M-CaAl-LDH) was successfully prepared by using cheap compounds such as CaO, AlCl3 and Fe2+/Fe3+ under the ultrasound-assisted co-precipitation condition. The crystal structure, surface morphology, and elemental compositions were characterized by XRD, FTIR-ATR, SEM–EDS, for the as-prepared LDH materials. The optimized results of removal effect for FA in the simulated sewage show that the removal rate was reached 92.15%, when 0.5 g M-CaAl-LDH was added to the simulated solution with initial pH = 7.5 after adsorption time of 60 min. As for CaAl-LDH before and after magnetic loadings, it is also found that the elimination capacity of M-CaAl-LDH is slightly lower than CaAl-LDH, which may be resulted from the loading of minor amounts of magnetic nano-Fe3O4 with poor removal ability.
Graphical Abstract









Similar content being viewed by others
Data and code availability
All data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdellaoui K, Pavlovic I, Bouhent M et al (2017) A comparative study of the amaranth azo dye adsorption/desorption from aqueous solutions by layered double hydroxides. Appl Clay Sci 143:142–150
Ahmed IM, Gasser MS (2012) Adsorption study of anionic reactive dye from aqueous solution to Mg–Fe–CO3 layered double hydroxide (LDH). Appl Surf Sci 259:650–656
Akbarnejad S, Amooey AA, Ghasemi S (2019) High effective adsorption of acid fuchsin dye using magnetic biodegradable polymer-based nanocomposite from aqueous solutions. Microchem J 149:e103966
Aoyun S, Xinrong L, Yongqiang Li (2021) Polyamine functionalized cotton fibers selectively capture negatively charged dye pollutants. Colloids Surf, A 623:e126666
Aranda M A G, Artioli I, Bier T, et al. (2017) Cementitious materials: Composition, properties, application. Walter de Gruyter GmbH & Co KG
Azin E, Moghimi H (2018) Efficient mycosorption of anionic azo dyes by Mucor circinelloides: surface functional groups and removal mechanism study. J Environ Chem Eng 6(4):4114–4123
Bhat IU, Anwar MN, Appaturi JN (2019) Polymer based palladium nanocatalyst for the degradation of nitrate and Congo red. J Polym Environ 27(7):1475–1487
Bhuyan D, Arbuj SS, Saikia L et al (2015) Template-free synthesis of Fe3O4 nanorod bundles and their highly efficient peroxidase mimetic activity for the degradation of organic dye pollutants with H2O2. New J Chem 39(10):7759–7762
Bin Hussein MZ, Yahaya AH, Shamsul M et al (2004) Acid fuchsin-interleaved Mg-Al-layered double hydroxide for the formation of an organic-inorganic hybrid nanocomposite. Mater Lett 58(3–4):329–332
Câmara AB, Sales RV, dos Santos Júnior CV, de Souza MA, de Longe C, Chianca TM, Dala Possa R, Bertolino LC, de Carvalho LS (2022) Eco-friendly adsorption of dye pollutants by palygorskite in aqueous effluents: Experimental and computational studies. Korean J Chem Eng 39(7):1805–1820
Dai X, Yi W, Yin C et al (2022) 2D–3D magnetic NiFe layered double hydroxide decorated diatomite as multi-function material for anionic, cationic dyes, arsenate, and arsenite adsorption. Appl Clay Sci 229:e106664
de Sá FP, Cunha BN, Nunes LM (2013) Effect of pH on the adsorption of Sunset Yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3). Chem Eng J 215:122–127
Dellamatrice PM, Silva-Stenico ME, Moraes LA, Fiore MF, Monteiro RT (2017) Degradation of textile dyes by cyanobacteria. Braz J Microbiol 48(1):25–31
Devaraj B, Thiruvengadam NJG, Rajamani R et al (2022) Microbial approaches for sustainable remediation of dye-contaminated wastewater: a review. Arch Microbiol 204(3):1–11
Dodoo-Arhin D, Buabeng FP, Mwabora JM, Amaniampong PN, Agbe H, Nyankson E, Obada DO, Asiedu NY (2018) The effect of titanium dioxide synthesis technique and its photocatalytic degradation of organic dye pollutants. Heliyon 4(7):e00681
Elmoubarki R, Mahjoubi FZ, Elhalil A, Tounsadi H, Abdennouri M, Sadiq MH, Qourzal S, Zouhri A, Barka N (2017) Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: preparation, characterization and application on textile dyes removal. J Mater Res Technol 6(3):271–283
Guanping P, Bei T, Xi Z (2021) Effect of preparation methods on the adsorption of glyphosate by calcined Ca–Al hydrotalcite. ACS Omega 6(24):15742–15749
He L, Zhi J, Li H et al (2022) Peroxymonosulfate activation by magnetic NiCo layered double hydroxides for naproxen degradation. Colloids Surf, A 642:e128696
Yu J, Zou J, Xu P, He Q (2020) Three-dimensional photoelectrocatalytic degradation of the opaque dye acid fuchsin by Pr and Co co-doped TiO2 particle electrodes. J Clean Prod 251(C):e119744
Kamali M, Esmaeili H, Tamjidi S (2022) Synthesis of zeolite clay/Fe–Al hydrotalcite composite as a reusable adsorbent for adsorption/desorption of cationic dyes. Arab J Sci Eng 47(5):6651–6665
Khan SB, Faisal M, Rahman MM, Jamal A (2011) Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci Total Environ 409(15):2987–2992
Khan MMR, Akter M, Amin MK et al (2018) Synthesis, luminescence and thermal properties of PVA-ZnO-Al2O3 composite films: towards fabrication of sunlight-induced catalyst for organic dye removal. J Polym Environ 26(8):3371–3381
Kheradmand A, Negarestani M, Kazemi S et al (2022) Adsorption behavior of rhamnolipid modified magnetic Co/Al layered double hydroxide for the removal of cationic and anionic dyes. Sci Rep 12(1):1–17
Kostić M, Najdanović S, Velinov N et al (2022) Ultrasound-assisted synthesis of a new material based on MgCoAl-LDH: characterization and optimization of sorption for progressive treatment of water. Environ Technol Innov 26:102358
Krishnan S, Chatterjee S, Solanki A et al (2020) Aminotetrazole-functionalized SiO2 coated MgO nanoparticle composites for removal of acid fuchsin dye and detection of heavy metal ions. ACS Appl Nano Mater 3(11):11203–11216
Lee JJ (2020) Analysis on isotherm, kinetic and thermodynamic properties for adsorption of acid fuchsin dye by activated carbon. Korean Chem Eng Res 58(3):458–465
Lei S, Wang S, Gao B et al (2020) Ultrathin dodecyl-sulfate-intercalated Mg-Al layered double hydroxide nanosheets with high adsorption capability for dye pollution. J Colloid Interface Sci 577:181–190
Li S, Bai H, Wang J et al (2012) In situ grown of nano-hydroxyapatite on magnetic CaAl-layered double hydroxides and its application in uranium removal. Chem Eng J 193:372–380
Li T, Du X, Deng J et al (2021) Efficient degradation of Rhodamine B by magnetically recoverable Fe3O4-modified ternary CoFeCu-layered double hydroxides via activating peroxymonosulfate. J Environ Sci 108:188–200
Liu Y, Wang N, Sun Z et al (2021) Selective adsorption of malachite green (MG) and fuchsin acid (FA) by ZIF-67 hybridized polyvinylidene fluoride (PVDF) membranes. Dalton Trans 50(25):8927–8937
Macedo-Miranda G, Martínez-Gallegos S, Ordoñez-Regíl E et al (2022) Triclosan removal on a MgAl hydrotalcite and its calcined product. Water Air Soil Pollut 233(2):1–14
Machrouhi A, Taoufik N, Elhalil A et al (2022) Patent Blue V dye adsorption by fresh and calcined Zn/Al LDH: effect of process parameters and experimental design optimization. J Compos Sci 6(4):115
Mansur AA, Mansur HS, Ramanery FP, Oliveira LC, Souza PP (2014) “Green” colloidal ZnS quantum dots/chitosan nano-photocatalysts for advanced oxidation processes: study of the photodegradation of organic dye pollutants. Appl Catal B: Environ 2014:158–159
Missau J, Bertuol DA, Tanabe EH (2021) Highly efficient adsorbent for removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH supported on Biochar. Appl Clay Sci 214:106297
Moosavi A, Amooey AA, Marzbali MH (2020) Extraordinary adsorption of acidic fuchsine and malachite green onto cheap nano-adsorbent derived from eggshell. Chin J Chem Eng 28(6):1591–1602
Ismail M, Akhtar K, Khan MI, Kamal T, Khan MA, Asiri M, A, Seo J, Khan SB, (2019) Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr Pharm Des 25(34):3645–3663
Mukurala N, Mokurala K, Suman S et al (2021) Synthesis of porous Cu2FeSnS4 particles via solvothermal process for removal of organic acid fuchsin dye pollutant from wastewater. Nano-Struct Nano-Objects 26:e100697
Nguyen HTT, Uyen DTT, Nguyen DC et al (2020) Characterization and evaluation of Ca/Al LDHs adsorbents synthesized by a one-step hydrothermal method for congo red removal. Mater Sci Forum 5996:195–200
Patil MR, Khairnar SD, Shrivastava VS (2016) Synthesis, characterization of polyaniline-Fe3O4 magnetic nanocomposite and its application for removal of an acid violet 19 dye. Appl Nanosci 6(4):495–502
Prashansa S, Suman P, Shambhavi R et al (2018) Green synthesis of silver nanoparticle capped with Allium cepa and their catalytic reduction of textile dyes: an ecofriendly approach. J Polym Environ 26(5):1795–1803
Radha A, Vishnu Kamath P, Shivakumara C (2005) Mechanism of the anion exchange reactions of the layered double hydroxides (LDHs) of Ca and Mg with Al. Solid State Sci 7(10):1180–1187
Renita AA, Kumar PS, Jabasingh SA (2019) Redemption of acid fuchsin dye from wastewater using de-oiled biomass: kinetics and isotherm analysis. Bioresour Technol Rep 7:e100300
Renita AA, Amarnath DJ, Duraikannu SL (2021) Synthesis of peanut-shell magnetized biocarbon for acid fuchsin dye removal. Mater Today: Proc 43:3075–3078
Saien J, Nasri M, Pourehie O (2022) Enhanced activation of persulfate by magnetic CuFe-layered double hydroxide nanocomposites under visible light irradiation for degradation of quinoline. J Iran Chem Soc 19(4):1515–1526
Shahabadi N, Razlansari M, Zhaleh H et al (2019) Antiproliferative effects of new magnetic pH-responsive drug delivery system composed of Fe3O4, CaAl layered double hydroxide and levodopa on melanoma cancer cells. Mater Sci Eng, C 101:472–486
Shahabadi N, Razlansari M, Zhaleh H (2022) In vitro cytotoxicity studies of smart pH-sensitive lamivudine-loaded CaAl-LDH magnetic nanoparticles against Mel-Rm and A-549 cancer cells. J Biomol Struct Dyn 40(1):213–225
Shen D, Liu J, Gan L et al (2018) Green synthesis of Fe3O4/cellulose/polyvinyl alcohol hybride aerogel and its application for dye removal. J Polym Environ 26(6):2234–2242
Smata A, Yoshimura C (2022) One-step synthesis of magnetic–layered double hydroxide and its application for oxytetracycline removal from water. J Environ Chem Eng 10(3):e107819
Suppaso C, Pongkan N, Intachai S et al (2021) Magnetically recoverable β-Ni (OH)2/γ-Fe2O3/NiFe-LDH composites; isotherm, thermodynamic and kinetic studies of synthetic dye adsorption and photocatalytic activity. Appl Clay Sci 213:e106115
Taheri S, Sedghi-Asl M, Ghaedi M et al (2023) Magnetic layered double hydroxide composite as new adsorbent for efficient Cu (II) and Ni (II) ions removal from aqueous samples: Adsorption mechanism investigation and parameters optimization. J Environ Manage 329:117009
Tong X, Yang Z, Xu P et al (2017) Nitrate adsorption from aqueous solutions by calcined ternary Mg–Al–Fe hydrotalcite. Water Sci Technol 75(9):2194–2203
Yang Z, Zhu L, Chen L (2019) Selective adsorption and separation of dyes from aqueous solution by core-shell structured NH2-functionalized UiO-66 magnetic composites. J Colloid Interface Sci 539:76–86
Yang B, Wei S, Tang K et al (2022) Study on the degradation performance of 2, 4-DCP by modified Co–Ni–Fe hydrotalcite. Catal Lett 152(2):383–397
Yongqin Hu, Chen H, Jia An et al (2022) Fe3O4-doped silk fibroin-polyacrylamide hydrogel for selective and highly efficient absorption of cationic dyes pollution in water. Nanotechnology 33(26):e265601
Yu Z, Li S, Zhang P et al (2017) Polymer-derived mesoporous Ni/SiOC (H) ceramic nanocomposites for efficient removal of acid fuchsin. Ceram Int 43(5):4520–4526
Zaatout AA, Khodary MA, Elkady MF (2015) Novel nano-zirconium antimonate as cation exchange material for organic dye pollutants purification. AmJ Appl Chem 3(3):46–46
Zhang P, Qian G, Shi H et al (2012) Mechanism of interaction of hydrocalumites (Ca/Al-LDH) with methyl orange and acidic scarlet GR. J Colloid Interface Sci 365(1):110–116
Zhang T, Xin X, Liu H et al (2020) Sol-gel preparation of spherical γ-Al2O3 with macro-mesopores as an efficient adsorbent for acid fuchsin. Micro Nano Lett 15(14):1017–1022
Zhang H, Yang S, Zhou X (2022) Mn-doped carbon dots as a visible-light-driven catalyst for degradation of acid fuchsin and malachite green. J Mater Sci: Mater Electron 33(7):4170–4183
Zhang H, Yang S, Zhou X (2022) Mn-doped carbon dots as a visible-light-driven catalyst for degradation of acid fuchsin and malachite green. J Mater Sci: Mater Electron 2022:1–14
Ziyat H, Elmzioui S, Naciri Bennani M et al (2021) Kinetic, isotherm, and mechanism investigations of the removal of nitrate and nitrite from water by the synthesized hydrotalcite Mg–Al. Res Chem Intermed 47(6):2605–2627
Acknowledgements
This study was supported by National Natural Science Foundation of China (21676264), Key R&D Program of Henan Province (scientific and technological) (212102310530), National Innovation and Entrepreneurship Training Program for college students of China (202013503006), Project of Scientific Research of Xinyang College (2022-XJLYB-001), Analysis and Testing Center of Xinyang College, Xinyang Municipal Key Laboratory of Green Synthesis for Nano Environment Friendly Materials and Key Disciplines (Chemistry) Program of Xinyang College.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by XJY and QQR. HL and XJY, and QQR analyzed data and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in this work.
Additional information
Editorial responsibility: Shahid Hussain.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Mao, L., Shuai, H. et al. Ultrasound-assisted synthesis of magnetic layer CaAl hydrotalcite composite for removal of fuchsin acid in simulated solution. Int. J. Environ. Sci. Technol. 21, 1591–1604 (2024). https://doi.org/10.1007/s13762-023-05052-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05052-y