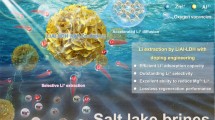
Abstract
An inorganic adsorbent with conveniently recovery performance is attractive in the treatment of uranium-containing wastewater. Herein magnetic Mg–Al layered double hydroxide composites (Mag-LDHs) and its calcination compound, Mag-LDHs (Mag-LDO), have been successfully prepared. Their physicochemical characteristic was analyzed with XRD, SEM, XPS, VSM, FT-IR, TGA, Zeta potential and N2 adsorption–desorption isotherms. The adsorption behavior of both adsorbents toward uranium were investigated. The solid–liquid separation could be conveniently achieved by external magnetic field. The maximum theoretical adsorption capacities toward U(VI) were 436.16 mg/g for Mag-LDHs and 317.71 mg/g for Mag-LDO. The adsorption capacity of Mag-LDHs and Mag-LDO retained 80.72% and 85.58% even undergoing five adsorption–desorption cycles. The study proposed Mag-LDHs and Mag-LDO as alternative uranium-specific adsorbents applicable in the acid wastewater (pH = 5.5).







Similar content being viewed by others
Data availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Fan M, Xe W, Song Q, Zhang L, Ren B, Yang X (2021) Review of biomass-based materials for uranium adsorption. J Radioanal Nuclear Chem 330:589–602
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117:13935–14013
Kiran, Bharti R, Sharma R. Effect of heavy metals: an overview. Mater Today Proc 2022, 51: 880–885
Guan X, Yuan X, Zhao Y, Bai J, Li Y, Cao Y, Chen Y, Xiong T (2022) Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J Colloid Interface Sci 612:572–583
Hu T, Gu Z, Williams GR, Strimaite M, Zha J, Zhou Z, Zhang X, Tan C, Liang R (2022) Layered double hydroxide-based nanomaterials for biomedical applications. Chem Soc Rev
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112:4124–4155
Zhao Y, Wang CJ, Gao W, Li B, Wang Q, Zheng L, Wei M, Evans DG, Duan X, O’Hare D (2013) Synthesis and antimicrobial activity of ZnTi-layered double hydroxide nanosheets. J Mater Chem B 1:5988–5994
Wu D, He F, Dai Y, Xie Y, Ling Y, Liu L, Zhao J, Ye H, Hou Y (2022) A heterostructured ZnAl-LDH@ZIF-8 hybrid as a bifunctional photocatalyst/adsorbent for CO2 reduction under visible light irradiation. Chem Eng J 446:137003
Nayak S, Parida K (2020) Superactive NiFe-LDH/graphene nanocomposites as competent catalysts for water splitting reactions. Inorganic Chem Front 7:3805–3836
Wei P-R, Cheng S-H, Liao W-N, Kao K-C, Weng C-F, Lee C-H (2012) Synthesis of chitosan-coated near-infrared layered double hydroxide nanoparticles for in vivo optical imaging. J Mater Chem 22:5503–5513
Li J, Cui H, Song X, Zhang G, Wang X, Song Q, Wei N, Tian J (2016) Adsorption and intercalation of organic pollutants and heavy metal ions into MgAl-LDHs nanosheets with high capacity. RSC Adv 6:92402–92410
Wang W-R, Li A, Mei W, Zhu R-R, Li K, Sun X-Y, Qian Y-C, Wang S-L (2015) Dexamethasone sodium phosphate intercalated layered double hydroxides and their therapeutic efficacy in a murine asthma model. RSC Adv 5:23826–23834
Mishra G, Dash B, Pandey S (2018) Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci 153:172–186
Tao Q, Reddy BJ, He H, Frost RL, Yuan P, Zhu J (2008) Synthesis and infrared spectroscopic characterization of selected layered double hydroxides containing divalent Ni and Co. Mater Chem Phys 112:869–875
Guan X, Yuan X, Zhao Y, Wang H, Wang H, Bai J, Li Y (2022) Application of functionalized layered double hydroxides for heavy metal removal: A review. Sci Total Environ 838:155693
Shandilya P, Sharma R, Arya RK, Kumar A, Vo D-VN, Sharma G (2021) Recent progress and challenges in photocatalytic water splitting using layered double hydroxides (LDH) based nanocomposites. Int J Hydrogen Energy 47(88):37438–37475
Li K, Liu H, Li Q, Yao W, Wu L, Li S, Wang Q (2022) The role of doped-Mn on enhancing arsenic removal by MgAl-LDHs. J Environ Sci China 120:125–134
Zhao XJ, Zhu YQ, Xu SM, Liu HM, Yin P, Feng YL, Yan H (2020) Anion exchange behavior of M(II)Al layered double hydroxides: a molecular dynamics and DFT study. Phys Chem Chem Phys 22:19758–19768
Bi R, Yin D, Lei B, Chen F, Zhang R, Li W (2022) Mercaptocarboxylic acid intercalated MgAl layered double hydroxide adsorbents for removal of heavy metal ions and recycling of spent adsorbents for photocatalytic degradation of organic dyes. Sep Purif Technol 289:120741
Wang C, Zhang R, Miao Y, Xue Q, Yu B, Gao Y, Han Z, Shao M (2021) Preparation of LDO@TiO2 core-shell nanosheets for enhanced photocatalytic degradation of organic pollution. Dalton Trans 50:17911–17919
Yao W, Yu S, Wang J, Zou Y, Lu S, Ai Y, Alharbi NS, Alsaedi A, Hayat T, Wang X (2017) Enhanced removal of methyl orange on calcined glycerol-modified nanocrystallined Mg/Al layered double hydroxides. Chem Eng J 307:476–486
Chen M, Li S, Li L, Jiang L, Ahmed Z, Dang Z, Wu P (2021) Memory effect induced the enhancement of uranium (VI) immobilization on low-cost MgAl-double oxide: Mechanism insight and resources recovery. J Hazard Mater 401:123447
Zhang S, Wang J, Zhang Y, Ma J, Huang L, Yu S, Chen L, Song G, Qiu M, Wang X (2021) Applications of water-stable metal-organic frameworks in the removal of water pollutants: a review. Environ Pollut 291:118076
Zhang J, Lu W, Zhan S, Qiu J, Wang X, Wu Z, Li H, Qiu Z, Peng H (2021) Adsorption and mechanistic study for humic acid removal by magnetic biochar derived from forestry wastes functionalized with Mg/Al-LDH. Sep Purif Technol 276:119296
Kevin S, Fadi C, Alexandre G, Sylvie BC, Dario T, Benoit PP (2019) Room temperature blocked magnetic nanoparticles based on ferrite promoted by a three-step thermal decomposition process. J Am Chem Soc 141(25):9783–9787
Zhu K, Chen C, Wang H, Xie Y, Wakeel M, Wahid A, Zhang X (2019) Gamma-ferric oxide nanoparticles decoration onto porous layered double oxide belts for efficient removal of uranyl. J Colloid Interface Sci 535:265–275
Behbahani ES, Dashtian K, Ghaedi M (2021) Fe3O4-FeMoS4: Promise magnetite LDH-based adsorbent for simultaneous removal of Pb (II), Cd (II), and Cu (II) heavy metal ions. J Hazard Mater 410:124560
Tao Q, Xie J, Li Y, Dai Y, Liu Z (2022) Effects of dry processing on adsorption of uranium on Mg-Al layered double hydroxides and calcined layered double oxides. J Radioanal Nucl Chem 331:4587–4600
Hou T, Yan L, Li J, Yang Y, Shan L, Meng X, Li X, Zhao Y (2020) Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent. Chem Eng J 384:123331
Dai Y, Lv R, Fan J, Peng H, Zhang Z, Cao X, Liu Y (2020) Highly ordered macroporous silica dioxide framework embedded with supramolecular as robust recognition agent for removal of cesium. J Hazard Mater 391:121467
Helal AA, Ahmed I, Gamal R, Abo-El-Enein S, Helal A (2022) Sorption of uranium (VI) from aqueous solution using nanomagnetite particles; with and without humic acid coating. J Radioanal Nucl Chem 331:3005–3014
Oguz E (2005) Adsorption characteristics and the kinetics of the Cr (VI) on the Thuja oriantalis. Colloids Surf, A 252:121–128
Duan J, Ji H, Xu T, Pan F, Liu X, Liu W, Zhao D (2021) Simultaneous adsorption of uranium (VI) and 2-chlorophenol by activated carbon fiber supported/modified titanate nanotubes (TNTs/ACF): Effectiveness and synergistic effects. Chem Eng J 406:126752
Chaudhary M, Singh L, Rekha P, Srivastava VC, Mohanty P (2019) Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine. Chem Eng J 378:122236
Yuvaraja G, Su M, Chen DY, Pang Y, Kong LJ, Subbaiah MV, Reddy GM (2020) Impregnation of magnetic-Momordica charantia leaf powder into chitosan for the removal of U (VI) from aqueous and polluted wastewater. Int J Biol Macromol 149:127–139
Zhao C, Liu J, Deng Y, Tian Y, Zhang G, Liao J, Sun Q (2019) Uranium (VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod 236:117624
El-Bohy MN, Abdel-Monem YK, Rabie KA, Farag NM, Mahfouz MG, Galhoum AA, Guibal E (2017) Grafting of arginine and glutamic acid onto cellulose for enhanced uranyl sorption. Cellulose 24:1427–1443
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Shao D, Li Y, Wang X, Hu S, Wen J, Xiong J, Marwani HM (2017) Phosphate-functionalized polyethylene with high adsorption of uranium (VI). ACS Omega 2(7):3267–3275
Qin X, Yang W, Yang W, Ma Y, Li M, Chen C, Pan Q (2021) Covalent modification of ZIF-90 for uranium adsorption from seawater. Microporous Mesoporous Mater 323:111231
Zhao S, Feng T, Feng L, Yan B, Sun W, Luo G, Wang N (2022) Rapid recovery of uranium with magnetic-single-molecular amidoxime adsorbent. Sep Purif Technol 287:120524
Li Y, Dai Y, Tao QQ, Gao Z, Xu L (2022) Ultrahigh efficient and selective adsorption of U(VI) with amino acids-modified magnetic chitosan biosorbents: Performance and mechanism. Int J Biol Macromol 214:54–66
Şenol ZM, Kaya S, Şimşek S, Katin KP, Özer A, Marzouki R (2022) Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: experimental and theoretical analyses. Int J Biol Macromol 209:1234–1247
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This work is financially supported by the Natural Science Foundation of Jiangxi Province of China (20224BAB203030, 20212ACB213001), the National Natural Science Foundation of China (22066001), and Jiangxi Province Innovation and Entrepreneurship Training Program for College Students (S202110405025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
It should be understood that none of the authors have any financial or scientific conflicts of interest with regard to the research described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, Q., Lin, S., Bai, T. et al. Surface magnetized MgAl-LDHs and MgAl-LDO with excellent adsorption capacity and convenient recovery for the removal of U(VI). J Radioanal Nucl Chem 332, 325–335 (2023). https://doi.org/10.1007/s10967-022-08740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08740-0