Abstract
This is the first study to investigate the antioxidant response of the phytoplankton community to heavy metal stress in the open sea. The levels of Cu, Zn, Cd, Mn, Pb, and Fe were determined in the phytoplankton community and water in Abu Talat (AT), and El Dekhella Harbour (DH), Alexandria coast during spring 2018 and autumn 2019. Furthermore, the changes in phytoplankton abundance, the bioconcentration factor (BCF), the metal pollution index (MPI), and the pollution load index (PLI) were used to assess pollution status. The phytoplankton density in the DH stations was 17–42 folds higher than in the AT. The order of BCF during spring was Fe > Zn > Pb > Cu > Mn > Cd. However, Fe > Zn > Mn > Cu > Pb > Cd was found in both areas during the autumn. The BCF, MPI, and PLI of the phytoplankton community revealed that DH is more polluted than AT. The phenol, 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and total antioxidant activity of the phytoplankton communities achieved higher values in DH than in AT stations, in response to heavy metal stresses. Principal component analysis was performed to follow pollution sources, suggesting that station 3 in both seasons was a hot spot because of stagnant water. A correlation matrix indicated that phenol and DPPH interacted positively in both locations during the two seasons. Analysis of variances with replicate tests on heavy metals in phytoplankton revealed significant seasonal differences across the two areas at a significance level < 0.05.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, heavy metal pollution in water bodies has attracted the attention of the scientific community, not only from the viewpoint of public health as related to water supplies but also to the depletion of aquatic organisms (Ismail and Ismail 2017; El Zokm et al. 2020, 2021b, 2022). Heavy metals have cumulatively harmful effects on aquatic species, such as plankton, macrophytes, and invertebrates at very low concentrations (Burada et al. 2006; Ismail and Ismail 2017). These heavy metals are introduced into the aquatic system from both natural and anthropogenic sources. The utilization of synthetic chemicals in agriculture is the main source of heavy metal pollution in an aquatic environment, leading to the deterioration of water quality and the depletion of aquatic biota (Garai et al. 2021).
One of the most important communities in aquatic ecosystems is phytoplankton. Phytoplankton species are the base of the food chain and are susceptible to the impacts of metal pollution (Permana and Akbarsyah 2019). They play an active role in the biogeochemical cycling of elements because of their high growth rate and biomass production, and they are characterized by a high bioaccumulation ability of heavy metals, so they are used as a bioindicator of metal pollution in an ecosystem (Rauf et al. 2019). Phytoplankton have a very high potential for heavy metal accumulation depending on both the hydrological and hydrochemical parameters of the area as well as the phytoplankton’s qualitative and quantitative characteristics (Nikolenko and Fedonenko 2020; Szymańska-Walkiewicz et al. 2022). Moreover, they may remarkably contribute to the transfer of heavy metals to higher trophic organisms, resulting in the destruction of their physiological systems and the mass death of biotas (Nikolenko and Fedonenko 2020). Phytoplankton play a unique role in the accumulation and distribution of heavy metals in aquatic biota, including available forms of heavy metals in biochemical cycles, promoting their transition from ionic to mineral forms and adsorbing metal compounds on the cell membrane (Nikolenko and Fedonenko 2020).
Heavy metals are regarded as stress factors for the growth of phytoplankton and cause physical and morphological changes in phytoplankton in a certain aquatic ecosystem (Permana and Akbarsyah 2019). Many heavy metals like Cd, Pb, Zn, Cu, Hg, and Cr have significant effects on physical, biochemical, and morphological characteristics as well as the growth of phytoplankton depending on the kinds and amounts of materials and environmental parameters (Jamers et al. 2013). It has been noted that a variety of environmental stressors, such as high light levels and heavy metals, induce the generation of reactive oxygen species (ROS) in microalgae (Coulombier et al. 2021). Algae can protect themselves by using two different antioxidant systems (enzymatic and non-enzymatic) (Ismail and Ismail 2017; Xiao et al. 2023). Phytoplankton are utilized as a bioindicator of heavy metals because of their comparatively high sensitivity to heavy metals (Permana and Akbarsyah 2019), so the assessment of heavy metal concentrations in plankton is crucial to evaluate the coastal area state.
Many studies have detected the occurrence of heavy metals in the different aquatic algal species (Ismail et al. 2017; El Zokm et al. 2021a; Ismail and Noaman 2022). But only a few studies refer to the heavy metals content in phytoplankton community in the field due to the difficulty of sampling and estimation process (Bahnasawy et al. 2009; Nikolenko and Fedonenko 2020; El Zokm et al. 2021a).
Multivariate statistical approaches, like the box and whisker plot, principal component analysis (PCA), correlation matrix and analysis of the test of variance (ANOVA), have been used as complementary techniques to describe the marine environment, focusing on spatiotemporal variations and shading light into variable interactions which giving deep insight into possible sources of pollutants affecting water quality (Yalcin 2020; El Zokm et al. 2021b, 2022). The box-whisker method is a standardized chart of showing the distribution of data based on a five-number summation [minimum, first quartile (Q1; 25%), second quartile (Q2; median 50%), third quartile (Q3; 75%) and maximum (El Zokm et al. 2022). PCA are widely used to reduce a large number of variables into a small number of significant factors; exploratory factor analysis is preferred in environmental studies by using the relationship between variables expressed as factor loading. Hot spot areas can be assessed by applying factor scores with the highest Eigenvalues (Shreadah et al. 2014; Yalcin 2020). Correlation analysis was performed through Spearman's rank-order to get the correlation coefficient. Positive correlations were interpreted to indicate comparable sources for the variables. Whereas, negative correlations were taken to indicate separate sources (Yalcin 2020). ANOVA was used to show whether the mean concentration of the studied parameter was significant or not according to the spatial and temporal distribution (El Zokm et al. 2021b).
The present study is devoted to evaluating the pollution level in the studied stations via determining the bioaccumulation factors of Cu, Zn, Cd, Mn and Pb in the water and phytoplankton community collected from ED and AT stations, Mediterranean Sea, Alexandria. The oxidative stress indices of algal communities were estimated to assess their ability to serve as biological indicators of pollution. As a comprehensive approach, boxplots, principal component analysis, correlation matrix, and analysis of variance are used to illustrate and discuss the estimation results.
Materials and methods
Study area
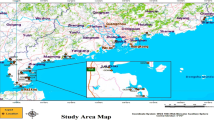
El-Dekhella harbour (DH) is located at the western part of El-Mex Bay at 29° 47′ and latitude 31° 10′ (Fig. 1). It has a surface area of about 12.5 km2, its water depth ranges between 6 and 19 m with an average of 12.4 m. Abu Talat (AT) region is a relatively unpolluted area (Long. 29o 70′ E and Lat. 31° 07′ N) that is fully exposed to wave action and has been temporarily affected by the freshwater from the east.
Phytoplankton collection
Surface water samples were collected by net (20 μm mesh size) from six stations in each DH and AT area during spring 2018 and autumn 2019. The collected samples were preserved immediately in Lugol’s solution and then preserved for subsequent examination. Estimation of phytoplankton abundance was carried out by the sedimentation method expressed as cell L−1 (APAH 1995). Subsamples were examined and enumerated with an inverted microscope at a magnification of 400, according to the method described by Lund et al. (1958). Standard texts were used for the identification of phytoplankton species according to Prescott (1970), Opute (1990, 2000), Tomas and Hasle (1997). The phytoplankton abundance was expressed as cell L−1. To determine heavy metal concentrations in phytoplankton communities, 500 L of surface water were filtered through a phytoplankton net to get representative samples. The filtrated material was digested using HNO3 and HClO4 (1:3 v/v), the samples were diluted up to an appropriate amount with deionized water (Schuhmacher and Domingo 1996), and were estimated by inductively coupled plasma atomic emission spectrometry for Fe, Mn, Cu, Zn, Pb and Cd and Milli-Q water was used for standard sample preparation. Heavy metals in the tested water areas are detected and published by El Zokm et al. (2021b). Phenol content of the phytoplankton community was determined according to Jindal and Singh (1975) with minor modifications, the cell pellets of the phytoplankton community were placed in 2.5 mL of absolute methanol for a period of 48 h at 4 °C in dark place. The cells were sonicated and the supernatant was separated by centrifugation (4500 g) for 10 min, 1.0 mL of methanolic extract was transferred to glass test tubes along with and 2 mL of sodium carbonate (2% v/v) were added and mixed well with 100 μL of Folin–Ciocalteu reagent (50%) then the mixture was vortexed and placed in the darkness for 1 h. The color developed was read at 695 nm using gallic acid (100 mg L−1) as standard. According to the Prieto et al. (1999) method, the total antioxidant capacity (TAC) of the phytoplankton methanolic extract was analyzed as equivalent to ascorbic acid standard (0.5 g/100 mL dist. H2O) and expressed as mg L−1. Sulphuric acid (0.6 M), sodium phosphate (28 mM), and ammonium molybdate (4 mM) were mixed in 250 mL with distilled water and known as (TAC) reagent. 0.3 mL of algal methanolic extract was added to 3 mL of TAC reagent and incubated at 95 °C for 90 min in caped tubes. Absorbance was read at 695 nm against blank. Antioxidant activity based on the DPPH free radical scavenging activity was estimated according to Molyneux (2004), 1 mL of methanolic extract was mixed with 1 mL methanolic solution of DPPH (0.1 mM). The reaction mixture was incubated for 30 min at room temperature; then, absorbance was recorded at 517 nm. The inhibition activity of free radicals was calculated according to the following formula:
where ODc is the absorbance of the control and ODs is the absorbance of the algal sample.
Quality assurance and quality control
Used glassware was washed with detergent before being soaked in 10% HNO3. A calibration technique for six external standards was evaluated for each determined parameter. An external standard has to be near to, but not identical to, the detection limit of the methodology. Fe, Mn, Zn, Cu, Pb, and Cd had detection limits and quantification limits of 0.045, 0.028, 0.012, 0.032, 0.012, and 0.010 g L−1, respectively. Each work shift's work calibration curve was confirmed by measuring one or more calibration standards. The recovery rate for each standard is around 92%. The coefficient variation was used to establish the data precision (10%).
Bioconcentration factor (BCF)
The bioconcentration level of heavy metals was calculated as a quantitative ratio of the metal concentration in the phytoplankton community to the metal concentration in the surrounding seawater (Ivanciuc et al. 2006; Ismail and Ismail 2017). equation 2 not 1.
where CR = mean metal concentrations in the phytoplankton community and CS = mean metal concentrations in seawater.
Metal pollution index (MPI)
To compare the total stored metals in the phytoplankton community from the studied sites, the MPI (Usero et al. 2005) was implemented.
where M is heavy metal concentration.
Pollution load index (PLI)
The Tomlinson PLI was used (Tomlinson et al. 1980) to assess the health with respect to the metals studied. PLI is used as an index of bioavailability of contaminants for organisms in coastal waters. It standardizes the data using the quotients obtained by dividing each concentration by a baseline concentration of the contaminant, i.e., the lowest concentration found during the study or reported in the literature. It is calculated using the following equation:
where CF (contamination factor) = Cmetal/Cbaseline.
Contamination level can be marked as follows: PLI > 1 polluted; PLI value < 1 unpolluted (Ismail and Ismail 2017; Seshan et al. 2010).
Statistical analyses
The employed software was IBM-SPSS Statistics version 22 and STATISTICA version 12.0. A correlation matrix with a correlation coefficient (n = 12 in each area for each season) at 95% and 99% confidence limit as well as principal component analysis (PCA) were applied to quantitatively analyze a sample of (12 × 3) variables distributed in each area. A box plot was also used to illustrate the data. An analysis of variance (ANOVA) test (two-way with replicates) was performed to study seasonal variation and parameter interaction. A one-way ANOVA test was also applied to predict if each parameter significantly or not significantly differed from 2018 to 2019 in each location. Principal component analysis was used to evaluate the phytoplankton quality index. Four factors with Eigenvalues higher > 1 (PC1, PC2, PC3, and PC4) with a cumulative covariance of 83.99% and 77.85% were used for the AT and DH regions, respectively. The phytoplankton quality index (PhQI) was calculated according to following formula:

whereas  is an eigenvalue, PCn is the factor score n is the number of effective principal components factor scores and ∑
is an eigenvalue, PCn is the factor score n is the number of effective principal components factor scores and ∑ are sum of eigenvalues. A high values of the principal component factor scores revealed that station can be considered as a hot spot (Shreadah et al. 2014; Akhtar et al. 2021).
are sum of eigenvalues. A high values of the principal component factor scores revealed that station can be considered as a hot spot (Shreadah et al. 2014; Akhtar et al. 2021).
Results and discussion
Distributions of heavy metals in the phytoplankton community
Comparative analyses of metal concentrations averaged in the collected phytoplankton community were occurred in the following abundance order: Fe > Zn > Pb > Cu > Mn > Cd in both studied areas in spring 2018 (Table 1). However, during autumn 2019, the average heavy metal concentrations in the phytoplankton community were sequenced as Fe > Zn > Mn > Cu > Cd > Pb and Fe > Zn > Mn > Cu > Pb > Cd at DH and AT, respectively. The concentrations of all metals were higher except for Pb, which may be due to the high accumulation rate of phytoplankton species in autumn. However, the bioaccumulation rate of heavy metals by phytoplankton is influenced by several factors, including the ability of a particular species to take up heavy metals and the season (Atici et al. 2012). Furthermore, the phytoplankton community in the DH was 17–42 times larger than in the AT. Additionally, the heavy metal concentrations of the phytoplankton community in DH were higher than those in AT, which may be related to increasing metal concentrations in water, resulting in increased metal concentrations in the biota (Deniseger et al. 1990). The concentration of heavy metals in DH has been confirmed to be two to sixfold higher than that in AT during assembly (El Zokm et al. 2021b).
There are no baseline data on heavy metals in phytoplankton communities, making this research incredibly rare in marine environments. Thus, there is a novelty in this study conducted along Alexandria's coastline in the coastal region of Egypt. The average concentration of metals like Zn, Pb, and Cd in the collected phytoplankton community (18.54, 1.6, 0.065 µg L−1) had the lower content compared to that (0.114, 0.118, 0.009 ppm) collected from the other two stations in the Mwanza Gulf, Lake Victoria, East Africa (Ogoyi et al. 2011), and also the heavy metal content of phytoplankton community from Zaporizhia reservoir (35, 3.5, 0.3 mg kg−1) (Nikolenko and Fedonenko 2020).
Assessing metal pollution in phytoplankton applying BCF, MPI, and PLI
The degree of impact of heavy metals on aquatic biota should be evaluated through metal content, patterns of entry, accumulation, and distribution in aquatic organisms (Nikolenko and Fedonenko 2020).
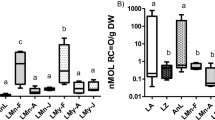
The BCF is defined as a ratio between metal concentrations in the phytoplankton community and water; thus phytoplankton could be used as a heavy metal bioindicator (Ismail and Ismail 2017). The results show that the BCF of metals by the phytoplankton community during the autumn season in both studied areas was the significantly higher than during the spring for all metals except Pb (Fig. 2). In general, a significant increase in BCF in DH over AT was observed. These significant variations may be related to the large surface of plankton biota, heavy metal concentration, the chemical groups (e.g., sulfonate, carboxyl, amino groups, and hydroxyl) on their algal cell walls, causing metal biosorption, and chelation and related to the morphology of the microalgae and season (Burada et al. 2014).
Pollution indices, such as MPI and PLI are efficient tools for assessing metal pollution and comparing sites and species (Yilmaz et al. 2021). The MPIph of DH phytoplankton ranged from 0.481 to 22.281 with an average value of 7.704, while AT ranged from 0.481 to 22.281 with an average value of 5.303, as shown in Table 2. The presence of anthropogenic activity in DH could explain the high mean (El Zokm et al. 2021b). Since phytoplankton species can accumulate metals from the surrounding medium, the MPI values of the resulting algal communities recommend these species as good pollution markers.
The PLI index was not previously applied to the phytoplankton community in the ecosystem. The estimated PLI values for the collected phytoplankton community were low, which ranged between 2.07E−28 and 3.49E−03 at AT stations and between 0.012 and 0.211 at DH stations during spring and autumn, respectively. Based on PLI indices of the phytoplankton community, both study areas are considered as unpolluted areas (Yalcin et al. 2019).
Statistical analysis
Multivariate analyses are recommended for environmental and monitoring research (Abdel Ghani et al. 2013; El Zokm et al. 2020, 2021a, 2021b, 2022). They can assist ecologists in discovering the structure of a set of data attributes and predicting a somewhat distant goal. These breakthroughs make it easier to comprehend the structure and function of even the most complicated theoretical investigations. Algae can protect themselves from the toxic and damaging impacts of heavy metals by trapping and accumulating pollutants in their polysaccharide walls (Bahnasawy et al. 2009). Most heavy metals, particularly Cu and Fe, induce oxidative stress in algae. Algal antioxidant systems play a crucial role in counteracting metal toxicity, whereas algae have developed a protective mechanism that consists of the preparation of antioxidant compounds that can minimize the concentration of these reactive oxygen species (Karthikeyan 2013). The activity of antioxidants is different between algal species; thus, there are metal-tolerant strains that have a more efficient antioxidant apparatus compared with metal-sensitive strains (Fogg 2001). Generally, many phytoplankton species act as natural sources of antioxidants due to their bioactive components (Karthikeyan 2013; El Zokm et al. 2021a). Algal cells tend to rapidly accumulate high concentrations of antioxidant substances, e.g., phenolic compounds and free proline in their cells. Phenol is non enzymatic antioxidant defense substance that has the ability to inactivate radicals by mono electronic transfer, and some can chelate the transition metals involved in the Fenton reaction, thus preventing the formation of the highly reactive hydroxyl radical (Ismail and Ismail 2017; Xiao et al. 2023). The ability of algae to tolerate various of biotic and abiotic stresses can be estimated by measuring their DPPH scavenging activity and TAC, which may be due to the abundance of phytoplankton, species, and heavy metal types, and contents in the water. The total phenolic content, TAC, and DPPH of the phytoplankton communities in the same area were significantly different among different stations, as shown in Fig. 3. The antioxidant results confirmed that the phytoplankton community in DK stations exhibited more oxidative stress abilities, which was significantly correlated with heavy metal concentrations and community composition. In both regions, all estimated DPPH and TAC showed higher levels in autumn than in spring, which may be due to the abundance of phytoplankton, species, and heavy metal types and contents in the water. In addition to the methanolic extracts of the collected phytoplankton communities exhibited the highest antioxidant activity, especially those collected from DK stations, might be due to the presence of high levels of phenol content, as shown in Tables 3 and 4.
Correlation matrix to study phyto-metal interactions
Tables 3 and 4 show the correlation coefficients of AT and DH results, respectively, and the correlation was considered significant at r = 0.576, stratified to show the relationship between phytoplankton abundance, antioxidant index, bioaccumulation factor, MPIPHY and PLIPHY. Phytoplankton abundance was significantly correlated with antioxidant index in two seasons in two study areas. The total phenolic content of the phytoplankton community was positively correlated with DPPH (r = 0.97 and r = 0.97 at AT, r = 0.87 and r = 0.95 at DH) and (r = 0.87 and r = 0.89 at AT, r = 0.85 at AT and r = 0.87at DH) in spring and autumn, respectively. Algal phenolic substances are known to be bioactive compounds because of their diverse biological activities such as antioxidant, antibacterial and antifouling activities (Kapoor et al. 2021; Ismail and Noaman 2022).
BFZn and BFFe were negatively correlated to MPIw (− 0.700 and − 0.617) and PLIw (− 0.703 and − 0.621) of AT stations, respectively. However, MPIph was strongly correlated with BFcu (0.96 and 0.99), BFzn (0.77 and 0.87), and BFFe (0.64 and 0.65) indicating that these metals contributed significantly to phytoplankton pollution at AT stations during the spring and autumn seasons. BFMn exhibited a positive relation with MPIph (r = 0.99) during the autumn season.
A different pattern appeared in the relationship between BAF and MPIph in DH (Table 4). BFCd (r = 0.899) and BFFe (r = 0.858) were highly correlated with MPIph during autumn. Zn is only positively correlated with MPIph (r = 0.91). This result reflects that the bioaccumulation of heavy metal in the phytoplankton was different according to the type of metal, algal species and seasons. Moreover, these relations suggest a high contribution of the detected metals in the phytoplankton community.
Significant correlations were found between several pairs of metal bioconcentration factors such as Cd–Mn (r = 0.86), Cu–Zn (r = 0.78), Pb–Fe (r = 0.75), Zn–Fe (r = 0.76) at AT areas during the spring and Cu–Zn (r = 0.83), Cu–Mn (r = 0.99), Zn–Mn (r = 0.82) during the autumn. This probably reflects the similar origin and similar chemical behavior of these elements.
Principal component analysis approach to discuss phyto‒metal interaction, sources, dynamic and pollution status
The PCA/FA approach (Table 5; n = 24 for each region) is used to reduce the number of variables by combining the correlating factors into new principal components and to identify the variables with the strongest impact on the phytoplankton abundance, BCF, TAC, DPPH, phytoplankton phenol content, heavy metals in water, and phytoplankton. The PCA evaluated how heavy metals interact with phytoplankton, taking into account their source and mode of action.
Four PCA were extracted by applying the Rotation Method: Varimax with Kaiser Normalization were applied to each region separately, accounting for 80.757% and 80.498% of the cumulative covariance in AT and DH, respectively. Although the two regions differ from each other and the PCA/FA approach was used separately, significant loading values for Cuph, Mnph, and Feph in PC1 were extracted from these two regions separately with 0.967, 0.973, and 0.906, respectively, in AT (PC1; 28.137%). However, PC1 in DH, which contributes to 29.238% has loading values of 0.976, 0.956, and 0.863 with Cuph, Mnph, and Feph respectively. PC1 in both regions seemed to have the same mode of action. It is evidenced that marine biota influences the chemical speciation of metals through the reduction and oxidation of redox active metals (Fe, Mn, and Cu) through various biological processes. These processes include the reduction of iron and copper by cell surface reductases or by biologically produced reducing agents (Tchounwou et al. 2012). Also, Cu is needed for Fe uptake and can metabolically substitute for Fe; colimitations can occur for Cu and Fe (Annett et al. 2008). The main proteins, superoxide dismutases (SODs), in eukaryotic algae are Fe-SOD, Mn-SOD, and Cu/Zn-SOD. Interestingly, PC1 in the AT data set only has high loadings of 0.982 and 0.900 with bioaccumulation factors of Cu and Mn, respectively, and the absence of these loadings in the DH region may be referred to as anthropogenic sources containing organic ligands as a scavenger to heavy metals. PC1 in the two regions could be named “bio redox factor”. PC2 in the two regions has 19.282% and 28.034% of variance in AT and DH, respectively, showing notorious loadings to DPPH and TAC with loadings − 0.902 and 0.84 in AT and 0.698 in DH for TAC. This factor may be explained by the assumption that phytoplankton communities with higher cell densities may be less exposed to environmental oxidative stress in the water which, may lead to lower concentrations of antioxidants produced by these microalgae. Moreover, oxidants play a dual role as both toxic and beneficial compounds. PC2 can be considered as an oxidative stress challenge factor, whereas the antioxidant activity of biota is related to its bioactive compounds, which are produced from normal cell metabolisms in situ or from external sources. When an overload of free radicals cannot be destroyed; their accumulation generates a phenomenon called “oxidative stress”.
PC3 was discussed separately in each of the two regions. It had a variance of 16.72% with high loadings to Pbw, Znw, and Few with 0.869, 0.852, and 0.797, respectively. It could be explained by the biogeochemical cycles of iron and zinc (naturally present in seawater), which are influenced by complexation to natural organics with stability constants of log KZn = 10–10.5 and log KFe = 12, and there is an anthropogenic source of lead that also has a high affinity to organic ligands, Fe and Zn act as cofactors. This factor could be designated as a complexity factor. However, PC3 in DH accounts for a 17.09% of variance with loading for CdP, PbP, and BCd at 0.888, 0.93, and 0.888, respectively, referring to the anthropogenic source. The cumulative contribution of PC4 in AT to Pb (0.862) and BPb (0.835) was 15.69%, because charged polysaccharides in phytoplankton cell walls have high affinity for Pb but not other metals (Ernst et al. 1992). PC4 in AT could be defined as lead phytoplankton uptake. PC4 in DH represented a variance of 16.33% with a high loading of BMn because Mn is an essential micronutrient involved in many cellular reactions such as photosynthesis and acts as an activator of several enzymes.
The calculation of principal component factor scores for the assessment of the PhQI according to the equation 5 (Table 6) revealed that the station (3) in DH in both seasons could be considered as hot spots (more polluted). This is consistent with the water quality characteristics of the station, which is stationary and not exposed to sea currents, allowing the accumulation of pollutants from DH's industrial activities as an important port in Alexandria. Station (3) is highly affected by PC3 in 2018 because of Cd and Pb accumulation in phytoplankton. However, it was affected by PC1 in 2019, pointing to the high levels of Cu, Mn and Fe in the phytoplankton community.
Analysis of the test of variance
In this study, a two-way ANOVA with replication was applied to examine the variation of parameters in each region individually (n = 24 for each region) to study seasonal variation in 2018–2019 and if the interaction between parameters is significant or non-significant. In AT; there was a significantly difference between two seasons (1.32E−05; p < 0.05). Also, a significant difference between the studied parameters (5.18E−22; p < 0.05) was recorded. Moreover, there was a significant difference in the interaction between the two factors (8.09E−18; p < 0.05) (Table 7). The same pattern of interaction was achieved in DH, with substantial. The same pattern of interaction was achieved in DH, with significant seasonal and parameter variation with p values of 1.93E−16 and 5.69E−44, respectively. There was also a significant interaction between parameters and seasons (p = 1.07E−41) (Table 8). However, a one-way analysis of variance (ANOVA) was conducted to highlight the seasonal variation of each parameter and evaluate the impact of metals (Balaguer et al. 2022). Only the phytoplankton community, DPPH%, and Zn showed significant seasonal variation in AT. Furthermore, n DH, there was a significant seasonal variation in every parameter except Fe, with no pb at all in AT and DH in 2019. Several factors have been reported to influence heavy metal accumulation in plankton, including the physico-chemical parameters of the water, the quantitative and qualitative composition of plankton species, heavy metals absorbance capacity, and the season (Burada et al. 2014).
Conclusion
It is crucial to investigate the effects of heavy metals on the phytoplankton at the cellular level in order to comprehend the process at play and the degree to which these metals will be transported to higher organisms via bioaccumulation. The results revealed significant differences in heavy metal concentrations and bioaccumulation in the phytoplankton community between different stations and seasons. The research proposed some statistical analyses that can work solely or combined with pollution indices to elucidate oxidative stress of heavy metals in phytoplankton community in two hotspot areas, namely, the DH (Port for cargo transportation) and AT region, which has tourist activities. The statistical framework provided a good conceptual understanding that is suitable for studies on sustainable development. It is apparent by applying PCA that phytoplankton communities have provided a dominant force in determining the distribution and chemistry of trace metals in seawater. Factor scores classified station (3) as the most polluted station during the two seasons. Through regular monitoring of heavy metals and long-term analysis of their effects on the diversity of biota, there should be an effort made to safeguard the water body from pollution in order to reduce environmental concerns. So, utilizing the phytoplankton community as a bioindicator, the results of this study could serve as the starting point for future assessments of metal contamination in order to inform decision-makers about the environmental situation and help them address potential issues. Continuous monitoring programs are advised for Egyptian coastal zones.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdel Ghani S, El Zokm G, Shobier A, Othman T, Shreadah M (2013) Metal pollution in surface sediments of Abu-Qir Bay and Eastern Harbour of Alexandria, Egypt. Egypt J Aquat Res 39(1):1–12. https://doi.org/10.1016/J.EJAR.2013.03.001
Akhtar N, Ishak MIS, Ahmad MI, Umar K, Md Yusuff MS, Anees MT, Qadir A, Almanasir YKA (2021) Modification of the water quality index (Wqi) process for simple calculation using the multi-criteria decision-making (MCDM) method: a review. Water (switzerland) 13(7):905. https://doi.org/10.3390/w13070905
Annett AL, Lapi S, Ruth TJ, Maldonado MT (2008) The effects of Cu and Fe availability on the growth and Cu:C ratios of marine diatoms. Limnol Oceanogr 53(6):2451–2461. https://doi.org/10.4319/lo.2008.53.6.2451
APAH (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association Inc., New York. References—Scientific Research Publishing. https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=1916575
Atici T, Obali O, Altindag A, Ahiska S, Aydin D (2012) The accumulation of heavy metals (Cd, Pb, Hg, Cr) and their state in phytoplanktonic algae and zooplanktonic organisms in Beysehir Lake and Mogan Lake, Turkey. Afr J Biotechnol 9(4):475–487. https://doi.org/10.4314/ajb.v9i4
Bahnasawy MH, Khidr AA, Dheina NA (2009) Assessment of heavy metals concentrations in water, plankton and fish of Lake Manzala, Egypt Egypt. J Aquat Biol Fish 13(2):117–133
Balaguer J, Koch F, Hassler C, Trimborn S (2022) Iron and manganese co-limit the growth of two phytoplankton groups dominant at two locations of the Drake Passage. Commun Biol 5(1):1–12. https://doi.org/10.1038/s42003-022-03148-8
Burada A, Jopa CM, Georgescu LP, Teodorof L, Nastase C, Seceleanu-Odor D, Negrea BM, Iticescu C (2006) Heavy metals accumulation in plankton and water of four aquatic complexes from Danube Delta area. AACL Bioflux 7(4):301–310
Burada A, Ţopa CM, Georgescu LP, Teodorof L, Năstase C, Seceleanu-Odor D, Negrea BM, Iticescu C (2014) AACL BIOFLUX heavy metals accumulation in plankton and water of four aquatic complexes from Danube Delta area 1,2. 7(4). http://www.bioflux.com.ro/aacl
Coulombier N, Jauffrais T, Lebouvier N (2021) Antioxidant compounds from microalgae: a review. Mar Drugs 19:549. https://doi.org/10.3390/md19100549
Deniseger J, Erickson LJ, Austin A, Roch M, Clark MJR (1990) The effects of decreasing heavy metal concentrations on the biota of Buttle Lake, Vancouver Island, British Columbia. Water Res 24(4):403–416. https://doi.org/10.1016/0043-1354(90)90222-R
El Zokm GM, Ibrahim MIA, Mohamed LA, El-Mamoney M (2020) Critical geochemical insight into Alexandria coast with special reference to diagnostic ratios (TOC/TN & Sr/Ca) and heavy metals ecotoxicological hazards. Egypt J Aquat Res 46(1):27–33. https://doi.org/10.1016/J.EJAR.2019.12.006
El Zokm GM, Ismail MM, El-Said GF (2021a) Halogen content relative to the chemical and biochemical composition of fifteen marine macro and micro algae: nutritional value, energy supply, antioxidant potency, and health risk assessment. Environ Sci Pollut Res 28:14893–14908. https://doi.org/10.1007/s11356-020-11596-0
El Zokm GM, Okbah MA, El-Shorbagi EK (2021b) Integrated approaches to assess water quality in two spots along the western Mediterranean Sea, Egypt. Chem Ecol J 37(6):493–514. https://doi.org/10.1080/02757540.2021.1892657
El Zokm GM, El-Said GF, El Ashmawy AA (2022) A comparative study, distribution, predicted no-effect concentration (PNEC) and contamination assessment of phenol with heavy metal contents in two coastal areas on the Egyptian Mediterranean Sea coast. Mar Environ Res 179:105687
Ernst WHO, Verkleij JAC, Schat H (1992) Metal tolerance in plants. Acta Bot Neerl 41(3):229–248. https://doi.org/10.1111/J.1438-8677.1992.TB01332.X
Fogg GE (2001) Algal adaptation to stress some general remarks. In: Rai LC, Gaur JP (eds) Algal adaptation to environmental stresses. Springer, Berlin, pp 1–19. https://doi.org/10.1007/978-3-642-59491-5_1
Garai P, Banerjee P, Mondal P, Saha NC (2021) Effect of heavy metals on fishes: toxicity and bioaccumulation. J Clin Toxicol. https://doi.org/10.35248/2161-0495.21.S18.001
Ismail GA, Ismail MM (2017) Variation in oxidative stress indices of two green seaweeds growing under different heavy metal stresses. Environ Monit Assess. https://doi.org/10.1007/s10661-017-5775-z
Ismail MM, Noaman NH (2022) Biochemical profile, nutritional value, and biological activities of Arthrospira platensis gomont. Hydrobiol J 58(6):56–80
Ismail MM, El Zokm GM, El-Sayed AAM (2017) Variation in biochemical constituents and master elements in common seaweeds from Alexandria Coast, Egypt, with special reference to their antioxidant activity and potential food uses: prospective equations. Environ Monit Assess. https://doi.org/10.1007/s10661-017-6366-8
Ivanciuc T, Ivanciuc O, Klein DJ (2006) Modeling the bioconcentration factors and bioaccumulation factors of polychlorinated biphenyls with posetic quantitative super-structure/activity relationships (QSSAR). Mol Divers 10(2):133–145. https://doi.org/10.1007/S11030-005-9003-3
Jamers A, Blust R, De Coen W, Griffin JL, Jones OAH (2013) Copper toxicity in the microalga Chlamydomonas reinhardtii: an integrated approach. Biometals 26(5):731–740. https://doi.org/10.1007/S10534-013-9648-9
Jindal KK, Singh RN (1975) Phenolic content in male and female Carica papaya: a possible physiological marker for sex identification of vegetative seedlings. Physiol Plant 33(1):104–107. https://doi.org/10.1111/j.1399-3054.1975.tb03774.x
Kapoor S, Singh M, Srivastava A, Chavali M, Chandrasekhar K, Verma P (2021) Extraction and characterization of microalgae-derived phenolics for pharmaceutical applications: a systematic review. J Basic Microbiol. https://doi.org/10.1002/jobm.202100458
Karthikeyan P (2013) In vitro antioxidant activity of marine diatoms. IOSR J Environ Sci Toxicol Food Technol 5(2):32–37. https://doi.org/10.9790/2402-0523237
Lund JWG, Kipling C, Le Cren ED (1958) The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11(2):143–170. https://doi.org/10.1007/BF00007865
Molyneux (2004) The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity.pdf (n.d.). Retrieved April 22, 2022, from https://www.researchgate.net/publication/237620105
Nikolenko Y, Fedonenko E (2020) Analysis of the content of heavy metals in phytoplankton of the Zaporizhia reservoir. ScienceRise Biol Sci 3(24):12–17. https://doi.org/10.15587/2519-8025.2020.210095
Ogoyi DO, Mwita CJ, Nguu EK, Shiundu PM (2011) Determination of heavy metal content in water, sediment and microalgae from lake Victoria, East Africa. Open Environ Eng J 4:156–161
Opute F (1990) Phytoplankton flora in the Warri/forcados estuary of southern Nigeria. Hydrobiologia 208(1):101–109. https://doi.org/10.1007/BF00008449
Opute FI (2000) Contribution to the knowledge of algae of Nigeria. I. Desmids from the Warri/Forcados Estuaries. Part II. The elongate baculiform desmids. J Limnol 59(2):131–155. https://doi.org/10.4081/jlimnol.2000.131
Permana R, Akbarsyah N (2019) Phytoplankton susceptibility towards toxic heavy metal cadmium: mechanism and its recent updates. World News Nat Sci 38:83–97
Prescott GW (1970) How to know the freshwater algae. Wm. C. Brown Company, Dubuque
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex. Anal Biochem 269(2):337–341
Rauf A, Javed M, Jabeen G (2019) Uptake and accumulation of heavy metals in water and planktonic biomass of the river Ravi, Pakistan. Turk J Fish Aquat Sci. https://doi.org/10.4194/1303-2712-V19_10_05
Schuhmacher M, Domingo JL (1996) Concentrations of selected elements in oysters (Crassostrea angulata) from the spanish coast. Bull Environ Contam Toxicol 56(1):106–113. https://doi.org/10.1007/s001289900016
Seshan BRR, Natesan U, Deepthi K (2010) Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. Int J Environ Sci Technol 7(2):291–306. https://doi.org/10.1007/BF03326139
Shreadah MA, Masoud MS, Khattab AM, El Zokm GM (2014) Impacts of different drains on the seawater quality of El-Mex Bay (Alexandria, Egypt). J Ecol Nat Environ 8(8):287–303. https://doi.org/10.5897/JENE2014.0465
Szymańska-Walkiewicz M, Glińska-Lewczuk K, Burandt P, Obolewski K (2022) Phytoplankton sensitivity to heavy metals in Baltic coastal lakes. Int J Environ Res Public Health 19(7):4131. https://doi.org/10.3390/IJERPH19074131
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012). Heavy metal toxicity and the environment. In: EXS, vol 101, pp 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Tomas CR, Hasle GR (1997) Identifying marine phytoplankton. Elsevier, Amsterdam, p 858
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters 33(1–4):566–575. https://doi.org/10.1007/BF02414780/METRICS
Usero J, Morillo J, Gracia I (2005) Heavy metal concentrations in molluscs from the Atlantic coast of southern Spain. Chemosphere 59(8):1175–1181. https://doi.org/10.1016/J.CHEMOSPHERE.2004.11.089
Xiao X, Li W, Jin M, Zhang L, Qin L, Geng W (2023) Responses and tolerance mechanisms of microalgae to heavy metal stress: a review. Mar Environ Res 183:105805
Yalcin F (2020) Data analysis of beach sands’ chemical analysis using multivariate statistical methods and heavy metal distribution maps: the case of moonlight beach sands, Kemer, Antalya, Turkey. Symmetry 2020(12):1538. https://doi.org/10.3390/sym12091538
Yalcin MG, Bilgen Coskun B, Nyamsari DG, Yalcin F (2019) Geomedical, ecological risk, and statistical assessment of hazardous elements in shore sediments of the Iskenderun Gulf, Eastern Mediterranean, Turkey. Environ Earth Sci 78:438
Yilmaz N, Ozyigit II, Demir HH, Yalcin IE (2021) Assessment on phytoplankton composition and heavy metal pollution in a drinking water resource: lake Terkos (Istanbul, Turkey). Desal Water Treat 225:265–274. https://doi.org/10.5004/dwt.2021.27221
Acknowledgements
The authors are grateful to the Maine Environmental Division, National Institute of Oceanography and Fisheries for the support of this work through the research plan titled “Variation in total phenol content in seawater, sediment, macrophyta and algae with special reference to antioxidant activity in studied biota 2018–2019”. The authors appreciate the efforts of Prof. Dr. Maged M. A. Hussein for his kind help in ANOVA test.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The article received no funding.
Author information
Authors and Affiliations
Contributions
MMI: Conceptualization, collection and identification of phytoplankton, methodology, writing, review, editing, and publishing. GMEZ: Visualization, methodology, statistics, writing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable: our manuscript does not report on or involve the use of any animal or human data or tissue.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Editorial responsibility: Rangabhashiyam S.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismail, M.M., El Zokm, G.M. Evaluation of the response of phytoplankton communities to heavy metal stresses using multi-statistical approaches, Alexandria coast, Egypt. Int. J. Environ. Sci. Technol. 20, 13595–13608 (2023). https://doi.org/10.1007/s13762-023-04914-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04914-9