Abstract
The use of aquatic biomass as potential sources for bioenergy production has attracted significant attention worldwide. Production of biogas and bioethanol from both marine and freshwater plants using same pre-treatment methods were evaluated and the results indicate that both processes can be potentially enhanced appropriate methods of pre-treatment. In this study, the effects of thermochemical and enzymatic pre-treatment of selected seaweeds and freshwater macrophytes for biogas and bioethanol production were investigated. It was found that methane biogas yield from the anaerobic digestion of selected aquatic plants was highly dependent on the plant species. For example, biomethane yields of 189, 195, 221, 234 mL/g volatile solids were obtained following anaerobic digestion of acid and enzymatic pre-treatment of Laminaria digitata, Sargassum fluitans, Eichhornia crassipies and Pistia stratiotes, respectively. Additionally, alcoholic fermentation by the yeast Saccharomyces cerevisiae (distiller’s strain) was carried out on aquatic plant hydrolysates and the highest ethanol yields (of over 4 g/L) were obtained from Eichhornia crassipies and Pistia stratiotes. Poor fermentation yields from Laminaria digitata, and Sargassum fluitans hydrolysates were attributed to the predominance of un-fermented rhamnose sugars in these plants. The findings demonstrate the importance of reliance on empirical data for each substrate when designing and operating anaerobic digestion and alcohol fermentation systems. The results show that the same pre-treatment methods can be used for both types of bioenergy production, i.e., biogas and bioethanol, from marine and freshwater plants, thereby enhancing the economic viability of both processes in industry-scale applications.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Apprehension over the continued use of fossils fuels for transportation and industrial uses poses significant environmental challenges particularly regarding greenhouse emissions, which contribute deleteriously to climate change (Sharma et al. 2021; Khan et al. 2021). The adverse impact from the use of fossil fuels has compelled researchers to focus on more sustainable energy sources. Biogas and bioethanol are renewable biomass-based energy sources that can ameliorate the aforementioned problems. Furthermore, anaerobic digestion technology has been reported to additionally mitigate poverty and hunger most especially in developing countries (Barbot et al. 2015).
Commercial biogas and bioethanol production around the world have been mainly limited to the use of traditional first and second-generation feedstocks, such as corn, wheat, sugar beet, sugar cane and energy grasses (Greene et al. 2020). However, competition for land and scarce water resources raises ethical concerns about the use of food-for-fuel (Akunna and Hierholtzer 2016).
In view of this, non-traditional (third-generation) feedstocks such as seaweeds and freshwater macrophytes are considered potential biomass sources for the production of bioethanol and biogas. This is due to the presence of readily hydrolysable sugars present in aquatic plants, with low amounts of cellulose and lignin content. Their use, however, is restricted by several factors including, their continuous supply for processing, and low biofuel yields (Wilkie and Evans 2010; Obata et al. 2020).
Improving yields from these feedstocks depends on the solubilisation of different organic components of aquatic biomass (Córdova et al. 2019). The first step in increasing the rate of hydrolysis is to apply pre-treatments to increase the biodigestibility by increasing the accessibility of the biomass constituents to hydrolytic enzymes and ultimately improving biofuel yield (Carrere et al. 2016).
Several new pre-treatment methods have been utilised on different lignocellulosic biomass sources such as ammonia fibre explosion (AFEX), catalysed steam explosion, high energy radiation (including microwave heating and ultrasound). Effective methods must be capable of liberating fermentable sugars from biomass with minimal formation of inhibitory compounds (Nasidi et al. 2015). The key barriers to the use of these methods are the scaling up of technologies to full commercial scale, which pose a serious challenge in terms of overall process economics. Therefore, the development of efficient, cost-effective and environmentally friendly pre-treatment processes is a prerequisite for the sustainability of the bioenergy process (Kostas et al. 2020).
Conventional dilute acid and enzyme pre-treatments have been employed due to the fact that the dilute acidic method has the ability to produce minimal inhibitory substances during hydrolysis when compared to concentred acid process, while the enzymatic step is considered environmentally friendly (Obata et al. 2015; Offei et al. 2018).
Previous research has reported the effects of different pre-treatment methods on seaweeds mostly from the temperate species (Jard et al. 2013; Barbot et al. 2016; Tedesco and Stokes 2017). For example, some researchers (17) have shown that Dilsea carnosa, Ulva lactuca and Laminaria digitata can produce up 4.7 g/L, 7.8 g/L and 3.2 g/ L of ethanol, respectively.
However, there is paucity of information on the effects on thermo-chemical and enzymatic pre-treatment on both tropical seaweeds and freshwater macrophytes for the production of both biogas and bioethanol. Hence, this study attempts to establish appropriate pre-treatment methods for the solubilisation of these aquatic plants and to better understand how pre-treatment affects process conditions and yields.
This study therefore investigated the effects of dilute acid treatment on enzyme hydrolysis on bioethanol and biogas production from selected species of these aquatic plants.
Materials and methods
Sample collection
Samples of the following brown macroalgal species (seaweeds) were collected as follows. Sargassum fluitans was harvested from Elecko beach at 6 26°’ 25.1’’N, 3° 50′ 49.7’’ E in Lagos, Nigeria. Laminaria digitata was harvested from the Broughty Ferry beach, Dundee, Scotland 56° 28′ 1.85 N, 2° 52′ 11.68’’ W, and used as a reference. Freshwater macrophytes, Eichhornia crassipes (water hyacinth) and Pistia stratiotes (water lettuce), were sourced from Modire/Vinikilang Benue river 9° 14′ 22.4’’ N, 12° 31′ 26.5’’ E in Yola, Adamawa State, Nigeria, and Epe river 6° 35′ 41.5’’ N, 3° 58′ 36.2’’ E in Lagos, Nigeria, respectively. The aquatic plants were dried, milled and stored in sealed containers at room temperature before they were used for the pre-treatment experiments decribed below.
Pre-treatment methods
Acid and heat pre-treatment
Powdered freshwater macrophytes and seaweeds (10% w/v) were weighed into 250 ml Erlenmeyer flasks in triplicate. 100 ml of dilute 1% HNO3 acid was added to each of the samples, then covered and autoclaved at 121 °C for 30 min and allowed to cool. After cooling, the pH was adjusted to 5.5 using drops of 10 M NaOH solution.
Enzymatic hydrolysis
Powdered acid digests of freshwater macrophytes and seaweeds (10% w/v) were pre-treated as described in 2.2.1. Subsequently, a cocktail of commercial enzymes from Novozymes (Denmark) biomass kit was used for the enzymatic hydrolysis at 50 °C with agitation at 150 rpm for 18 h. Table 1 shows the characteristics of the enzymes as provided by the manufacturer.
Anaerobic digestion reactor setup
Anaerobic digestions were carried out in 500 mL glass bottles. The glass bottles possessed thick rubber septum as caps to allow for maintenance during sampling. 50 mL pre-treated feedstock samples were diluted with 150 mL of the non-growth media and seeded with 100 mL of anaerobically digested sludge to make up 300 mL of culture volume. All culture reactors were incubated at 37 °C for 50 days, after initially purging the headspace with nitrogen gas for 2 min. Two sets of controls were adopted: blank samples containing only the inoculum and medium were set up to discount the methane production from the inoculum, and un-treated samples containing inoculum and raw samples were set-up in order to determine the effectiveness of the pre-treatment methods on anaerobic digestion. Anaerobically digested sewage sludge was used as inoculum.
Ethanol fermentation reactor setup and preparation of seed culture
The fermentation setup was carried out in 250 Erlenmeyer flasks after both acid and enzymatic pre-treatments. The hydrolysates were sterilised by autoclaving and separated into 100 mL aliquots. Accordingly, aliquot hydrolysate was inoculated with S. cerevisiae yeast (Distillmax distillers’ strain from Lallemand Biofuels and Distilled Spirits) at a pitching rate of 107 cell/mL into flasks and plugged with cotton wool. Subsequently, the fermentation was carried out at 30 °C with shaking at 100 rpm. 5 mL samples were collected every 24 h and centrifuged at 6700 rpm for 15 min, to determine specific sugars and ethanol concentrations until the end of the fermentation at 150 h. The yeast strain was cultured on yeast extract peptone dextrose (YEPD) agar plates consisting of the following compounds: glucose10g/L, bacteriological peptone 10 g/L, yeast extracts 3 g/L and technical agar 20 g/L.
Single colonies of yeast from a YEPD plate were used for the preparations of seed cultures. For all the experiments yeast inocula were prepared in glucose synthetic media consisting of Yeast Nitrogen Base 6.9 g/L, (NH4)2 SO4 3.4 g/L, potassium hydrogen phthalate 3.06 g/L, yeast extract 0.4 g/L and glucose 40 g/L with pH adjusted to 5.5. All experiments were conducted in 500 ml Erlenmeyer flasks; 300 mL glucose synthetic media was incubated with yeast colonies at 30 °C on a rotary shaker at 100 r.p.m for 48 h. Cells from the 48-h culture were used for the fermentation experiments.
Analytical methods
The soluble chemical oxygen demand (SCOD) was measured using the potassium dichromate method, and sample concentrations determined using cuvette test kits LCK 014 (Hach-Lang, USA) as reported by Hierholtzer et al. (2013). The extractive protein content was analysed using the Coomassie Bradford (Bradford 1976), protein assay. 2 g of sample was mixed with 18 ml of 2 M NaOH and incubated at 65 °C for 1 h. Samples were centrifuged and the supernatant used for the protein assay. The carbohydrate content was analysed using a method adapted from the National Renewable Energy Laboratory (Washington, USA), a more detailed procedure can be found from NREL/TP-570-42,618. The concentration of ammonium nitrogen was determined using cuvette test kits LCK 304 (Hach-Lange, USA). Total, volatile solids and ash content were determined according to the standardised methods by oven-drying at 105 °C and incinerating at 550 °C as previously described (APHA 1998). Volatile fatty acids (VFA) concentrations of the anaerobic cultures were determined by esterification method (Montgomery et al. 1962). To quantify the amount of reducing sugars in the supernatants of seaweeds and freshwater macrophytes hydrolysates, a method developed by Miller (1959) was adopted using dinitro salicylic acid (DNS). The methane content of the biogas was analysed with GC (Hewlett-Packard 5890 series II). Ultimate methane yields were determined based on the method of Hansen et al. (2004). Sugars and ethanol concentration were determined by High performance Liquid Chromatography (HPLC) with a 300 mm X 7.8 mm REZEX ROA-Organic Acid column: Schimadzu® Prominence®.
Results and discussion
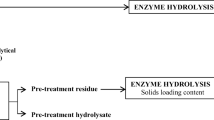
Freshwater macrophytes and seaweeds offer alternative to terrestrial crops to generate energy from. However, their conversion to different energy sources have been hampered by low yields and lack of cost-effective treatment methods to aid their solubilisation. To find cost-effective processes for harnessing values from these plants, selection of appropriate conversion process that could be used in biorefinery has been proposed in this study and shown in Fig 1. Therefore, this study investigated the effects of various pre-treatment methods for potential application in biorefinery (Fig. 1). Table 2 shows the characteristics of the feedstocks used in the study.
Effects of pre-treatment on SCOD production
According to Akunna (2018), the dissolved or soluble chemical oxygen demand (SCOD) content of raw materials is a crucial parameter used to evaluate the effectiveness of pre-treatment processes for anaerobic digestion. Therefore, in Fig. 2, the influence of dilute acid and enzyme pre-treatment on SCOD solubilisation was evaluated. An enhanced solubilisation of the aquatic biomass was observed when both dilute acid and enzyme pre-treatment method were tested. The highest SCOD values were observed in the acid plus enzymatic treatment for all feedstocks. For example, in L. digitata SCOD solubilisation increased from 14 to 22% for dilute acid and acid plus enzyme treatment, respectively. For S. fluitans 40% and 52%, for P. stratiotes 37% and 57%, for E. crassipes 48% and 67% increases were recorded compared to the untreated samples. A similar increase in SCOD solubilisation was observed when Ulva specie was treated with HCL and NaOH (Jung et al. 2016). These results demonstrate that both dilute acid and enzyme-based pre-treatment methods can effectively break down these aquatic plants for enhanced bioenergy production.
Effects of acid and enzymatic pre-treatment on the production of reducing sugars
As shown in Fig. 3, dilute 1% HNO3 and enzymatic pre-treatment led to higher reducing sugar production in the feedstocks. Differences were observed in all the feedstocks when dilute HNO3 pre-treatment is compared to the enzymatic hydrolysis. Results show increments in reducing sugar production of 37%, 36%, 54% and 46% in S. fluitans, L. digitata, P. stratiotes and E. crassipes, respectively. These results seem to suggest that the enzymatic step may be necessary to convert structural carbohydrate polymers to monosaccharides in these substrates. Interestingly, enzymatic hydrolysis was observed to be more effective in the freshwater macrophytes than seaweeds. This result suggests that acid pre-treatment might have facilitated the release of more inhibitory substances such as phenols, salts and 5-Hyroxymethylfurfural (HMF). Phenols at 2 g/L, salts at 10 g/L and 5-Hyroxymethylfurfural at 6 g/L have been reported as inhibiting to the fermentation processes (Kostas et al. 2020; Obata et al. 2016; Alzate-Gaviria et al. 2021).
The amount of specific sugars released after the enzymatic hydrolysis of the 1% HNO3 pre-treatment is shown in Fig. 4. There were distinctive differences of sugar types within the samples, as follows: glucose was the dominant sugar in the freshwater macrophytes 78% and 67% in E. crassipes and P. stratiotes, respectively; rhamnose was the dominant sugar within the seaweed substrates in L. digitata 65%, and S. fluitans 56%. These results indicate that for efficient utilisation of the different dominant sugars in the substrate for bioenergy production, selection of appropriate microbes that can effectively utilise the different sugars is vital.
Effects of thermo-chemical and enzymatic pre-treatment on biogas and VFA production
To determine the effects of different pre-treatment methods on the anaerobic biodegradability of each freshwater macrophytes and seaweed species used in the study, anaerobic digestion of both un-treated and pre-treatment samples were carried out over a 50-day period. Results from Figs. 5 and 6 show higher VFA production in both acid and acid plus enzyme pre-treated L. digitata and E. crassipes cultures. This indicates the effectiveness of the pre-treatment methods for both feedstocks. However, the VFA concentrations recorded in L. digitata and E. crassipes cultures for both acid and acid plus enzyme treated cultures were above the recommended limit of 2000 mg/L (Figs. 5 and 6) which could have affected methanogenesis (Nkemka and Murto 2010). The pH of the reactors fell below 6.0 during the experiment and no methane production was observed in the first 10 days. The pH in the reactors was then corrected to 7.5 with 10 M NaOH. Gradual methane production was recorded from reactors after pH adjustment. Manns et al. (2016), in their study, noted that pre-treatment did not increase the surface area of L. digitata as the macroalgae consisted of flat leaves.
Furthermore, this result clearly shows the inseparable link between VFA reduction and methane production. A possible explanation for the high VFA reported in pre-treated L. digitata and E. crassipes cultures is the high SCOD as shown in Fig. 2. This provided relatively high quantities readily biodegradable organic material for microbes to break down and subsequently led to accelerated acidogenesis stage that ultimately caused acid accumulation. In S. fluitans and P. stratiotes reactors for both acid and acid plus enzyme pre-treated cultures, VFA inhibition was not detected, due probably to their relatively low production rate of SCOD as evidenced in Fig 2. The increase in cumulative methane production was recorded by Day 8 and 5, respectively. Bird et al. (1990), in their research, concluded that S. fluitans is a poor feedstock for biogas production with a low yield of 120 mL/g VS, due to fibre like materials in the cell wall of the seaweed. However, an enhanced methane production was recorded in this study of 195 mL/g VS. This result clearly shows that an appropriate pre-treatment step is needed to increase the solubilisation of this seaweed species.
Generally, methane yields of pre-treated S. fluitans cultures when compared to those of un-treated cultures show a percentage increment of + 5% and + 40% for heat plus acid and heat plus acid plus enzymes, respectively. By comparison, P. stratiotes show a percentage increment of + 8% and + 18% for heat plus acid and heat plus acid plus enzymes, respectively.
Overall, results showed enhanced acid production caused by the pre-treatment of each of the pant species used in the study, which could ultimately affect the methane yield. Effective methane production may require longer retention times to ensure sufficient conversion of VFA. Alternatively, a two/three-stage reactor system is recommended for substrates with high SCOD in order to prevent VFA accumulation and subsequent inhibition of the methanogenic stage in the anaerobic digestion process (Zhou et al. 2017). This observation necessitates reliance on empirical data for each specific substrate when designing and operating an anaerobic digestion system.
Effects of thermo-chemical and enzymatic pre-treatment bioethanol production
S. cerevisiae is the most commonly used yeast strain in alcoholic fermentation processes (Walker and Stewart 2016). In Fig. 7, the ability to ferment various sugars produced by seaweed and freshwater macrophytes was examined. There were variations in peak ethanol production among different aquatic plants. S. cerevisiae showed a high affinity for the fermentation of glucose, which is predominant in the hydrolysates of freshwater macrophytes, producing 4.7 g/L (0.20 g/g) and 4.1 g/L (0.27 g/g) ethanol in E. crassipes and P. stratiotes, respectively. However, lower ethanol yields were recorded in the algal species, with S. fluitans and L. digitata only producing 1.5 g/L (0.20 g/g) and 3.0 g/L (0.10 g/g) ethanol. Due to the nature of the unique sugars in seaweeds species, S. cerevisiae in unable to utilise these for fermentation (Obata et al. 2016). Therefore, non-conventional yeast species such as Kluveromyces marxianus and Schefferomyces stipitis are required to effectively utilise diverse sugars, as shown in Table 3.
For freshwater macrophytes (Mishima et al. 2008) reported higher ethanol production for E. crassipes and P. stratiotes 10.1 g/L and 11.3 g/L, respectively. However, lower ethanol yields were recorded in relation to this study. This may be due to different fermentation strategies coupled with nutrient supplementations.
These findings indicate that the diverse sugars within seaweeds and freshwater macrophytes hydrolysates could be effectively utilised if appropriate yeast species and fermentation strategies are adopted.
Conclusion
This study has shown that both pre-treatment methods can enhance hydrolysis and methane production differently in selected marine plants. For biogas production, pre-treatment enhanced anaerobic biodegradation as evidenced by accumulation of acids (VFA). This shows that pre-treatment can accelerate hydrolysis and acidogenesis, which if not appropriately taken into account, could lead to accumulation of VFA and consequently inhibition of methanogenesis. For the bioethanol production process, low yields were recorded in some of the plant species used in this study. This is likely due to the presence of inhibitors within the hydrolysate that limit maximum utilisation of sugars by yeast in the fermentation process. Therefore, optimisation of fermentation approaches and conditions by adopting simultaneous saccharification and fermentation, nutrient supplementation and detoxification of hydrolysates will likely maximise ethanol yields from these plant species.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adams JMM, Toop TA, Donnison IS, Gallagher JA (2011) Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour Technol 102(21):9976–9984
Akunna JC (2018) Anaerobic waste-wastewater treatment and biogas plants: a practical handbook. CRC Press. https://doi.org/10.1201/9781351170529
Akunna JC, Hierholtzer A (2016) Co-digestion of terrestrial plant biomass with marine macro-algae for biogas production. Biomass Bioenerg 93:137–143. https://doi.org/10.1016/j.biombioe.2016.07.106
Alzate-Gaviria L, Domínguez-Maldonado J, Chablé-Villacís R, Olguin-Maciel E, Leal-Bautista RM, Canché-Escamilla G, Caballero-Vázquez A, Hernández-Zepeda C, Barredo-Pool FA, Tapia-Tussell R (2021) Presence of polyphenols complex aromatic “Lignin” in Sargassum spp. from Mexican Caribbean. J Mar Sci Eng 9(1):6. https://doi.org/10.3390/jmse9010006
American Public Health Association (1998) Standard methods for the examination of water and wastewater. In: Clesceri LS, Greenberg AE, Eaton AD (eds) 20th edn. American Public Health Association, American Wastewater Association, Water Environmental Federation, Washington DC, USA
Barbot YN, Al-Ghaili H, Benz R (2016) A review on the valorization of macroalgal wastes for biomethane production. Mar Drugs 14(6):120. https://doi.org/10.3390/md14060120
Barbot YN, Falk HM, Benz R (2015) Thermo-acidic pretreatment of marine brown algae Fucus vesiculosus to increase methane production—A disposal principle for macroalgae waste from beaches. J Appl Phycol 27(1):601–609. https://doi.org/10.1007/s10811-014-0339-x
Bird KT, Chynoweth DP, Jerger DE (1990) Effects of marine algal proximate composition on methane yields. J Appl Phycol 2(3):207–213. https://doi.org/10.1007/BF02179777
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I (2016) Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Biores Technol 199:386–397. https://doi.org/10.1016/j.biortech.2015.09.007
Córdova O, Passos F, Chamy R (2019) Enzymatic pretreatment of microalgae: cell wall disruption, biomass solubilisation and methane yield increase. Appl Biochem Biotechnol 189(3):787–797. https://doi.org/10.1007/s12010-019-03044-8
Greene JM, Gulden J, Wood G, Huesemann M, Quinn JC (2020) Techno-economic analysis and global warming potential of a novel offshore macroalgae biorefinery. Algal Res 51:102132. https://doi.org/10.1016/j.agal.2020.102032
Hansen TL, Schmidt JE, Angelidaki I, Marca E, la Cour JansenMosbækChristensen JHTH (2004) Method for determination of methane potentials of solid organic waste. Waste Manage 24(4):393–400. https://doi.org/10.1016/j.wasman.2003.09.009
Hierholtzer A, Chatellard L, KIeransAkunnaCollier MJCPJ (2013) The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J Appl Microbiol 114(4):964–973. https://doi.org/10.1111/jam.12114
Jard G, Dumas C, Delgenès JP, Marfaing H, Sialve B, Steyer JP, Carrère H (2013) Effect of thermochemical pretreatment on the solubilization and anaerobic biodegradability of the red macroalga Palmaria palmata. Biochem Eng J 79:253–258. https://doi.org/10.1016/j.bej.2013.08.011
Jung H, Baek G, Kim J, Shin SG, Lee C (2016) Mild-temperature thermochemical pretreatment of green macroalgal biomass: effects on solubilization, methanation, and microbial community structure. Biores Technol 199:326–335. https://doi.org/10.1016/j.biortech.2015.08.014
Khan MAN, Shiekh Z, Parveen S, Ahmed S, Irfan M, Gauttam R, Shah AA, Jamal A, Khan S, Badshah M (2021) Production of bioethanol and biogas from Spirodela polyrhiza in a biorefinery concept and output energy analysis of the process. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02066-9
Kostas ET, White DA, Cook DJ (2020) Bioethanol production from UK seaweeds: investigating variable pre-treatment and enzyme hydrolysis parameters. Bioenergy Res 13(1):271–285. https://doi.org/10.1007/s12155-019-10054-1
Manns D, Andersen SK, Saake B, Meyer AS (2016) Brown seaweed processing: enzymatic saccharification of Laminaria digitata requires no pre-treatment. J Appl Phycol 28(2):1287–1294. https://doi.org/10.1007/s10811-015-0663-9
Miller GL (1959) Modified DNS method for reducing sugars. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Mishima D, Kuniki M, Sei K, Soda S, Ike M, Fujita M (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour Technol 99(7):2495–2500. https://doi.org/10.1016/j.biortech.2007.04.056
Montgomery H, Dymock JF, Thom N (1962) The rapid colorimetric determination of organic acids and their salts in sewage-sludge liquor. Analyst 87(1041):949–955. https://doi.org/10.1039/AN9628700949
Nasidi M, Agu R, Deeni Y, Walker G (2015) Improved production of ethanol using bagasse from different sorghum cultivars. Biomass Bioenerg 72:288–299. https://doi.org/10.1016/j.biombioe.2014.10.016
Nkemka VN, Murto M (2010) Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J Environ Manage 91(7):1573–1579. https://doi.org/10.1016/j.jenvman.2010.03.004
Obata O, Akunna J, Bockhorn H, Walker G (2016) Ethanol production from brown seaweed using non-conventional yeasts. Bioethanol 2(1):134–145. https://doi.org/10.1515/bioeth-2016-0010
Obata O, Akunna JC, Walker G (2015) Hydrolytic effects of acid and enzymatic pre-treatment on the anaerobic biodegradability of Ascophyllum nodosum and Laminaria digitata species of brown seaweed. Biomass Bioenerg 80:140–146. https://doi.org/10.1016/j.biombioe.2015.05.001
Obata O, Ditchfield A, Hatton A, Akunna J (2020) Investigating the impact of inoculum source on anaerobic digestion of various species of marine macroalgae. Algal Res 46:101–803. https://doi.org/10.1016/j.algal.2020.101803
Offei F, Mensah M, Thygesen A, Kemausuor F (2018) Seaweed bioethanol production: a process selection review on hydrolysis and fermentation. Fermentation 4(4):99. https://doi.org/10.3390/fermentation4040099
Sharma S, Jha PK, Panwar A (2021) Production of bioethanol from wheat straw via optimization of co-culture conditions of Bacillus licheniformis and Saccharomyces cerevisiae. Discover Energy 1(1):1–8. https://doi.org/10.1007/s43937-021-00004-4
Tedesco S, Stokes J (2017) Valorisation to biogas of macroalgal waste streams: a circular approach to bioproducts and bioenergy in Ireland. Chemicke zvesti 71(4):721. https://doi.org/10.1007/s11696-016-0005-7
Walker GM, Stewart GG (2016) Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2(4):30. https://doi.org/10.3390/beverages2040030
Wilkie AC, Evans JM (2010) Aquatic plants: an opportunity feedstock in the age of bioenergy. Biofuels 1(2):311–321. https://doi.org/10.4155/bfs.10.2
Zhou J, Yang J, Yu Q, YongXieZhangWeiJia XXLPH (2017) Different organic loading rates on the biogas production during the anaerobic digestion of rice straw: a pilot study. Biores Technol 244:865–871. https://doi.org/10.1016/j.biortech.2017.07.146
Acknowledgements
This work was supported by funding from the Nigerian Petroleum Technology Development Fund (PTDF).
Author information
Authors and Affiliations
Contributions
FJ: performed writing original draft, conceptualisation, formal analysis and investigation. JA-performed writing, review, editing, conceptualisation and supervision. GW- performed writing, review, editing, conceptualisation and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jelani, F., Walker, G. & Akunna, J. Effects of thermo-chemical and enzymatic pre-treatment of tropical seaweeds and freshwater macrophytes on biogas and bioethanol production. Int. J. Environ. Sci. Technol. 20, 12999–13008 (2023). https://doi.org/10.1007/s13762-023-04843-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04843-7