Abstract
Invasive species usually grow in human-disturbed habitats including urban areas where they face numerous pollutants, including metals that attract special attention due to their non-degradability and high accumulation potential. In this study we evaluated the contents of potentially toxic metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in the invasive species Solidago gigantea. The strategy adopted by S. gigantea in response to excess trace metals in the soil was assessed. Metal contents were determined using atomic absorption spectrometry in leaves, stems and roots of S. gigantea collected from 30 sites located in areas affected by various human activities. Metal concentrations (total and bioavailable fraction) were also determined in corresponding soil samples. Results showed that S. gigantea was able to inhabit strongly polluted sites. High Bioaccumulation Factor but low Translocation Factor values for Cd, Cu, Cr, Fe, Ni show that S. gigantea takes up metals from soil but reduces their transport to the aboveground parts and could be classified as a metal-tolerant species with exclusion strategy. The Bioaccumulation Factor values for Cd, Cu, Cr, Pb and Zn were remarkably higher in plants growing in areas characterized by low metal concentrations in soil compared to the ones affected by industry, with high metal concentrations. This suggests that S. gigantea that grows in polluted areas can reduce the uptake of potentially toxic metals. Regardless of the limited transport of metals to the aboveground organs, the content of metals in S. gigantea leaves differed between areas differing in human impact and the species can be used as a biomonitor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of toxic and potentially toxic substances are released into the environment due to numerous human activities, including municipal, industrial, commercial and agricultural operations (Wong et al. 2006). Metals are the pollutants that attract special attention (Ali et al. 2013; Liu et al. 2019; Luo et al. 2019). Their emissions vary according to the land use type as well as factors, such as geographical, climatic and sociological conditions (Sáňka et al. 1995). However, pollution is often increased in urban areas (Wong et al. 2006; Liu et al. 2019; Luo et al. 2019) and it has been shown that industrial activity and traffic have a strong impact on the contamination level (Sáňka et al. 1995). As metals are non-degradable, they accumulate in the environment (Ali et al. 2013), especially in soil, considered a regional sink for pollutants (Ferreira et al. 2018).
As plants are immobile and strongly bound to their habitats they have to cope with the toxic effects of metals present in the environment. Consequently, they developed three types of response to excess trace metals in soil: reduction in uptake or exclusion of metals, stress resistance, and accumulation (Baker 1981; Yang et al. 2007). According to Wójcik et al. (2014), most plants do not regulate metal uptake to the roots and restrict translocation to the shoots; these are known as excluders. The species characterized by active uptake and translocation of metals within their tissues are known as accumulators. When proportional relationships between metal levels in soil and in plant parts can be seen, the species are known as indicators. One species may employ quite diverse mechanisms for different metals (Baker 1981). Understanding the status of elements in soil–plant systems is necessary to evaluate the risk that pollutants will enter plant tissues and the food chain (Nworie et al. 2019). Based on each of the above-mentioned mechanisms, plants are useful for certain applications in phytoremediation or assessment of the pollution level. Excluders may be used in phytostabilization, accumulators in phytoextraction, while indicators in the estimation of metal bioavailability and of the state of the environment (Wójcik et al. 2014).
Solidago gigantea Aiton (Giant goldenrod) is a perennial herb from the Asteraceae family (Balicevic et al. 2015). The species is of much interest because of its invasiveness and a threat posed to biodiversity (Scharfy et al. 2009; Szymura and Szymura 2013). In fact, all non-native Solidago species are considered highly successful invaders in Europe (Chmura et al. 2015; Szymura et al. 2016) and they rapidly colonize Asia and Australia (Tokarska-Guzik et al. 2012). Solidago gigantea is native to North America and was introduced to Europe in the eighteenth century as an ornamental plant. The species is adapted to a wide range of soil conditions, spreads quickly and occupies anthropogenic, semi-natural as well as natural ecosystems. It prefers ruderal habitats, riversides, roadsides and abandoned agricultural areas (Scharfy et al. 2009; Szymura et al. 2018). It is used as a medicinal plant (an urological, anticancer and anti-inflammatory agent) as well as a supplementary source of nectar and pollen for honeybees (Szymura and Szymura 2015; Szymura et al. 2018). Similarly as other invasive plants S. gigantea can survive in harsh conditions and pose a threat to natural plants there; therefore, the knowledge about its ecology in polluted sites is important (Bobulska et al. 2018).
The invasiveness of S. gigantea is associated for example with production of numerous wind-dispersed seeds, easy germination on a wide range of soils as well as fast vegetative growth (Balicevic et al. 2015; Chmura et al. 2015; Pisula and Meiners 2010). In addition to the negative impact on biodiversity invasive species may disturb biogeochemical cycles in colonized habitats, e.g., through increased absorption of nutrients in topsoil (Szymura and Szymura 2015). A study by Bobulska et al. (2019) showed that soil enzymes and physicochemical properties were altered in habitats invaded by S. gigantea. On the other hand, contents of trace metals and the bioaccumulation potential of this plant were rarely examined (Nowińska et al. 2012; Wójcik et al. 2014). According to Wójcik et al. (2014) and Kowalska et al. (2012) S. gigantea can inhabit soils strongly polluted with Ba, Cd, Cr, Cu, Ni, Zn and Pb and characterized by lower trace metal contents compared to other species growing in the same areas. However, Wójcik et al. (2014) stated that despite the significant biomass and ubiquity, the Goldenrod was not suitable for phytoextraction in waste dumps due to low metal contents. To the best of our knowledge, the literature lacks comprehensive information on the concentration and translocation of metals in Solidago gigantea as well as its possible application in biomonitoring studies. On the other hand there are a few biogeochemical studies on Solidago canadensis, a closely related species, which showed that it can tolerate metal pollution in soils (Antonijević et al. 2012; Bielecka and Królak 2019a,b; Yang et al. 2007 and 2008, Xiang et al. 2010) and discussed its possible application in bioindication and phytoremediation (Bielecka and Królak 2019a). In the study of Bobulska et al. (2018) the genus Solidago was proved to be an accumulator of Pb, Cu and Cr in polluted areas, which suggests that species of this genus have interesting biogeochemical features expected in species used in environmental management. S. gigantea has spread throughout Europe since the nineteenth century and it currently is permanently established in many natural and anthropogenic habitats (Tokarska-Guzik et al. 2012; Szymura et al. 2018); therefore, its suitability for application, e.g., in bioindication as well as its contribution to metal transfer to food chains are worth consideration. In addition, biogeochemical studies may shed more light on the explanation of its invasiveness: it has already been suggested that increased environmental pollution provides more opportunities for the invasion of alien species (Li et al. 2021) and that invasive plants in metal-polluted urban environments may be even more successful than in natural sites due to the better adaptation or tolerance to stressful conditions compared to native plants (Yang et al. 2007; Sołtysiak et al. 2014). According to Li et al. (2021) invasive plants showed self-protective mechanisms when exposed to heavy metals and metal pollution may favor plant invasion due to the widespread higher tolerance of invasive plants to heavy metals compared to native species. On the other hand, accumulation of anthropogenic pollutants by invasive species has been reported to influence the weed biological control system. Sorensen et al. (2009) observed that Mn, Se and Cr were transferred from Tamarix ramosissima plants to Diorhabda elongata beetles (the biological control agent) and the growth of the larvae of D. elongata was significantly reduced by Se contamination.
The aims of this work is to describe the strategy of S. gigantea in response to metal pollution and to propose its possible applications in environmental management. Therefore, contents of potentially toxic metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in the roots, stems and leaves of S. gigantea growing in different types of human-disturbed environments were studied to assess the bioaccumulation and translocation ability of S. gigantea. It is hypothesized that: (1) S. gigantea can inhabit areas with high metal concentrations in soil; (2) in response to excess trace metals in soil, the species can reduce the uptake of metals or their translocation to aboveground organs. In addition, based on the results the first attempt was made to assess if S. gigantea can be used as a bioindicator of metal pollution in urban and industrial areas.
The samples for the study were collected in 2018 in the urban areas of the Wrocław agglomeration (Lower Silesia, SW Poland), and the research was carried out at the University of Wrocław (Wrocław, Poland).
Material and methods
Sampling design
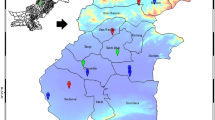
Thirty sampling sites were designated in and around the Wrocław agglomeration (Fig. 1). Each sampling square was at least 250 m away from the others. Location (geographical coordinates), characteristics, land use and detailed description of the study sites are given in Table 1. The climate of the region is considered mild, in the category of temperate climates, transitional between the maritime and continental type. The area is one of the warmest regions of Poland with the annual mean temperature about 8 °C and the vegetative season of 215–225 days. The lowest temperatures as well as precipitation totals are usually recorded in January, while the highest in July. The annual rainfall in the region is 550–600 mm. Weak westerly winds prevail (Głowicki et al. 2005; Fabiszewski 2005).
In September of 2018, ten mature, healthy-looking plants of S. gigantea were collected randomly in each sampling site within a square of 25 m2 and mixed as one sample. Five topsoil samples from a depth of 0–15 cm were also collected from each square and mixed into one sample (about 1 kg).
Chemical analysis
Soil samples were dried at room temperature, sieved through a 2-mm sieve to remove coarse material and homogenized in a Pulverisette two laboratory grinder (FRITSH GmbH Mahlen und Messen, Germany). Soil pHH20 was measured potentiometrically (soil to distilled water ratio of 1:2.5) using an HI9107 microprocessor pH meter (Hanna Instruments, USA). Soil samples (0.5 g) were digested with nitric acid (5 mL, 65%) and hydrogen peroxide (0.5 mL, 30%) in an open system, with temperature set to 95 °C. The digests were diluted to 50 mL with deionized water and then analyzed for Fe, Mn and Zn using flame atomic absorption spectrophotometry (FAAS) and for Cd, Cr, Cu, Ni and Pb using electrothermal atomic absorption spectrometry (ETAAS) with a GF3000 Graphite Furnace (AVANTA PM AAS, GBC Scientific Equipment, Australia). The bioavailable fraction of metals in soils was determined after DTPA-extraction. The extracts were prepared by shaking air-dried soils with extracting solution (0.005 M DTPA, 0.01 M CaCl2 and 0.1 M TEA adjusted to pH 7.3), filtered (Benton Jones 2001) and analyzed for metals using the AAS methods described above.
Any adhering materials were removed from the plant surface, and plants were subsequently washed in deionized water, separated into leaves, stems and roots, dried at 80 °C to a constant weight and homogenized in a POLYMIXPX-MFC 90 D laboratory mill (Kinematica AG, Switzerland). The plant samples (0.5 g) were digested in an open system using the methods described above for soil samples (Kalra 2019). The digests were analyzed for Fe, Mn and Zn using FAAS and for Cd, Cr, Cu, Ni and Pb using ETAAS.
All the elements were tested against atomic absorption spectrometry standard solutions (Sigma-Aldrich, USA) and blanks. The results were calculated on a dry-weight basis. The accuracy of the methods was checked against certified reference materials: IPE 952 Grass (mixture)/Poaceae (Wageningen Evaluating Programs for Analytical Laboratories, The Netherlands) for plants and METRANALQCM 31 light sandy soil (trace elements) (ANALYTIKA® spol. s r.o., Czech Republic) for soil samples. The recovery rates (percent) for IPE 952 were: Cd 97.5 Cr 93.3, Cu 103, Fe 101, Mn 97.3, Ni 94.2, Pb 97.3, Zn 98.4 and for METRANALQCM 31: Cd 96.8, Cr 102, Cu 104, Fe 99.2, Mn 97.0, Ni 106, Pb 107, Zn 94.7.
Data analysis
The study sites were grouped into three categories according to the local sources of pollution and land use type to analyze differences in metal concentrations and bioaccumulation depending on the level of environmental pollution: (1) study sites located near a non-ferrous metal refinery and ironworks (numbers 1–5 and 11–13); (2) study sites located near a heat and power plant and a slag heap of a former ferrochrome steel plant (number 6–10); (3) study sites in non-industrial areas (residential and agricultural) (numbers 14–30) (Table 1).
Shapiro–Wilk’s W-test was used to check the normality of data. Since the variables were not normally distributed nonparametric tests were chosen. The differences in metal contents in the organs of S. gigantea as well as in soils between the three groups of study sites were determined by non-parametric Kruskal–Wallis ANOVA with the median post hoc test. The relationship between the concentration of metals in plants and in soil was evaluated using the Spearman correlation coefficient (Zar 1999). In all the performed tests the significance level was set at 0.05.
After the Box-Cox transformation of the variables, the matrices of the contents of six elements (Cd, Cr, Cu, Mn, Fe, Zn) in plant leaf samples from 30 sites were subjected to principal component and classification analysis (PCCA) (Legendre and Legendre 1998).
To evaluate the ability of S. gigantea to take up elements from soil, the Bioaccumulation Factor (BF) (Bidar et al. 2009) was calculated as the ratio of metal concentrations in plant roots to their DTPA-extracted concentrations in soil. Concentrations of bioavailable metals were preferred over total concentrations as neutral soil extraction is considered a better indicator of metal phytoavailability than total metal contents in soil (Stefanowicz et al. 2016; Dambiec et al. 2017). The translocation Factor (TF) was calculated as the ratio of metal concentrations in the stems/leaves to those in the roots to assess the mobility of metals in the plant and internal metal transport (Bonanno et al. 2018; Bidar et al. 2009).
All calculations were done with Statistica 13 (StatSoft Inc. 2016).
Results and discussion
Metal content in soils
Soil pH ranged from 5.7 to 7.7 (mean of 6.9), within the range noted in typical habitats occupied by S. gigantea (Bielecka 2017; Szymura and Szymura 2013). Total metal contents in the soil in the study sites indicated wide ranges of metal levels that characterized S. gigantea stands (Fig. 2). Groups of study sites differed significantly in Cd, Cu, Cr, Fe, Pb and Zn concentrations (Kruskal–Wallis ANOVA, p < 0.05). Cadmium, Cu, Pb and Zn contents in soils from groups 1 and 2 were significantly higher than in group 3 (median test, p < 0.05). Cadmium, Cu and Pb contents in all soils from groups 1 and 2 were higher than the geochemical background in Poland (0.18, 7.1, 9.8 mg kg−1, respectively) (Czarnowska 1996). In addition, Zn contents in all sites in group 1 and the maximum values in groups 2 and 3 exceeded the geochemical background for Zn (30 mg kg−1) (Czarnowska 1996). On average, the Cr content in group 2 was the highest and significantly higher than in group 3 (median test, p < 0.05) but in each group maximum values only were higher than values typical for the geochemical background (27 mg kg−1) (Czarnowska 1996). Iron and Mn contents were relatively high compared to the mean soil content in Poland and the geochemical background (1200 and 289 mg kg−1, respectively) (Czarnowska 1996; Kabata-Pendias 2011) in all groups of study sites and their values in group 1 were significantly the highest (median test, p < 0.05). Nickel contents did not differ between the groups of study sites and in each group maximum values only were higher than the values typical for the geochemical background (10.2 mg kg−1) (Czarnowska 1996). Metal contents in soil that exceed background values indicate either the presence of geochemical anomalies or the enrichment of elements caused by human activity (Gałuszka and Migaszewski, 2011). Highly elevated total soil concentrations of Cu, Pb, Zn and Cd (Fig. 2) reported in the study sites affected by the industrial processing of copper products and their alloys with zinc and lead (study sites 1–5 in group 1) were consistent with the previous studies of Meinhardt (2016) who reported Cd, Cu, Pb and Zn soil pollution in the vicinity of this industrial plant. Relatively high concentrations of Zn and the highest Pb contents were also noted in soil near the zinc smelter (study sites 11–13 in group 1). The main cause of soil pollution with Zn and Pb in this region was atmospheric precipitation of metalliferous dusts emitted by the zinc smelter (Cuske et al. 2013). Increased Cr contents in soil in group 2 were associated with the former activity of the ferrochrome smelter and the presence of a slag heap (Karczewska and Bortniak 2008). Average metal contents in soils in group 3 were not higher (Cd, Cu, Cr, Mn, Ni) or only slightly higher (Pb and Zn) than the background values given above. It can be assumed that the study sites in group 3 were mostly unpolluted and characterized by metal contents similar to the geochemical background.
Total (tot) and bioavailable (bioav) metals content [mg kg−1 DW] in soil in each group (Gr 1, Gr 2 and Gr 3) of the study sites. The values with different letters are significantly different (the median test, p < 0.05): italic font indicates differences between groups in terms of total metal content and bold font indicates differences between groups in terms of bioavailable metal content
The total concentration of metals in soils is a useful indicator of the extent of soil contamination but the environmental risk from metal and plant accumulation depends on the bioavailability of the elements (Kandziora-Ciupa et al. 2017; Liu et al. 2019). The bioavailable metal contents in the soil (Fig. 2) showed similar relations between the groups of study sites: the average metal concentrations in group 3 were significantly lower than in groups 1 and 2 (for Cr, Pb and Zn) or lower than in group 1 (for Cd and Cu). No significant differences between the groups were found for Fe, Mn and Ni contents.
The results of this study showed that soil was strongly polluted in many S. gigantea sites. The ability of this species to grow in strongly contaminated soils was previously reported by Wójcik et al. (2014) who studied plants that spontaneously inhabited Zn-Pb waste deposits characterized by extremely high total Zn, Pb and Cd concentrations (7300–171,790, 1390–2226, 66–1464 mg kg−1, respectively). Similarly, Kowalska et al. (2012) reported that S. gigantea colonized industrial areas characterized by relatively high trace metal contents in soil (in mg kg−1): Ba > 300, Cr 140, Cu 110, Ni 80 and Pb 70. In addition, Bielecka (2017) noted that other species of the Solidago genus tolerated high metal contents in soil. The tolerance to a wide range of soil pollution allows the species to colonize many different habitats and may give an advantage over other, especially native, species. According to Yang et al. (2008) S. canadensis, a species of the same genus, became a better competitor under Pb pollution because the facilitating efficiency of mycorrhizae on nutrient acquisition was promoted by high Pb contents in soil (300 and 600 mg/kg soil).
Metal contents and translocation in Solidago gigantea
The Bioaccumulation Factor (BF) was calculated to estimate metal relocation from soil to plant roots (Table 2). Factor values higher than one indicate a plant’s ability to take up metals from soil (Galal and Shehata 2015). For Cd, Cu, Cr, Fe, Ni and Zn BFs were greater than 1 (except for Cu and Zn in group 1) (Table 2), thus confirming the high uptake efficiency for these metals from soil into the roots of the species studied (Eid et al. 2012). These observations are consistent with Bobulska et al. (2018) who showed that Cr and Cu were the elements efficiently removed by the Solidago genus in polluted areas. However, noteworthy was the fact that in the present study the BF values for Cd, Cu, Cr, Pb and Zn were remarkably higher in group 3 compared to other groups. This result indicates that the BF values were lower in the areas with higher metal concentrations in soil which suggests that S. gigantea was able to reduce the uptake efficiency of these elements from soil with high metal concentrations. Similar relationships between the concentrations of Pb and Zn in soil and their accumulation in S. canadensis, a closely related species, were observed by Bielecka and Królak (2019a). Furthermore, Yang et al. (2007) suggested that S. canadensis in Pb polluted soil had an ability to exclude Pb or reduce the uptake of Pb. The lowest BF values, lower than 1 in all groups of study sites, were observed for Mn and Pb. Despite the fact that Mn is regarded as an easily bioavailable metal (Kabata-Pendias 2011; Weiss et al. 2003), low Mn uptake was previously observed for a few species of wild herbaceous plants growing near power stations (Mandzhieva et al. 2016) and Plantago major growing in heavy-traffic affected soil (Galal and Shehata 2015), which confirmed that some plants can limit the uptake of easily bioavailable metals.
The contents of Cd, Cu, Cr, Mn, Pb and Zn in S. gigantea organs differed significantly between the groups of study sites (Kruskal–Wallis ANOVA, p < 0.05) (Fig. 3). Similar relations between the groups as for soil were observed. Cadmium contents in roots, stems and leaves were the highest in group 1 compared to groups 2 and 3 (median test, p < 0.05). Chromium contents in leaves were significantly higher in group 2 than in groups 1 and 3; in roots they were significantly higher in group 2 than in group 1; and in stems they were significantly higher in group 2 than in group 3. Cu and Zn contents in all organs in group 1 were significantly higher than in group 3. Lead contents in roots in groups 1 and 2 were significantly higher than in roots in group 3. Differences in Mn contents were noted in aboveground organs only: leaves in group 3 contained significantly more Mn than in groups 1 and 2, and in the case of stems significant differences were noted between groups 3 and 1. In some study sites metal contents in plant organs were highly elevated when compared to the average contents in plants given by Markert (1992): Cd > 0.5 mg kg−1 in roots in all study sites in groups 1 and 3 as well as in stems and leaves in group 1; Cu > 20 mg kg−1 in roots in all groups and in leaves in group 1; Cr > 1 mg kg−1 in roots in all groups as well as in leaves in group 2; Zn > 150 mg kg−1 in all organs in group 1; and Fe > 200 mg kg−1 in roots in all groups. The results suggest that metal contents in the organs of S. gigantea reflect the level of environmental metal pollution. Elevated metal contents were observed in the organs of S. gigantea growing in the vicinity of industrial plants, e.g., Cd, Cu, Zn near a processing facility for copper products and their alloys (Meinhardt 2016) or the zinc smelter (Cuske et al. 2013); Cr in plants near the slag heap of the former ferrochrome smelter (Karczewska and Bortniak 2008). The cause of the relatively high Mn contents in plants in group 3 can be the use of fertilizers in agricultural areas (Reimann and de Caritat 1998; Adriano 2001).
Metals content [mg kg−1 DW] in the organs of S. gigantea in each group (Gr 1, Gr 2 and Gr 3) of the study sites. The values with different letters are significantly different (the median test, p < 0.05): regular font indicates differences between groups in terms of metal content in roots; bold font indicates differences between groups in terms of metal content in stems and italic font indicates differences between groups in terms of metal content in leaves
Regardless of the above-mentioned differences between the study sites, there were significant differences in element contents between the organs in all groups of study sites (Kruskal–Wallis ANOVA, p < 0.05). The concentrations of Cd, Cr, Cu, Fe, Ni and Pb were the highest in roots, while of Zn and Mn in leaves. The lowest Cr, Cu, Fe, Mn, Ni contents were observed in stems. High concentrations in roots suggest limited mobility and translocation of these metals taken up by S. gigantea. Accordingly, the Translocation Factor (TF), which illustrates internal metal transport in plants (Galal and Shehata 2015), was below 1 for the majority of metals (Table 2). The limited mobility of elements between roots and shoots characterizes excluder plants with the mechanism of resistance to trace metal pollution (Baker 1981; Mandzhieva et al. 2016). In many plant species avoidance mechanisms accounting for resistance could be, e.g., binding of metals by mycorrhizal fungi or binding to root cell walls (Lambers et al. 2008). A similar scheme of metal accumulation has been reported for another invasive species (S. canadensis) whose underground organs contained more Cu and Pb than aboveground organs (Antonijević et al. 2012; Bielecka and Królak 2019b; Yang et al. 2008). Metal sequestration in roots enables plants to continue uninhibited growth, and it is an important mechanism of metal tolerance (Jasion et al. 2013; Page et al. 2006). On the other hand, Mn and Zn were transported from roots to the aboveground organs of S. gigantea, as shown by TF values > 1 (Table 2). Similar behavior was previously reported by Zheljazkov et al. (2008) for five medicinal plant species growing in polluted soil: metal concentrations in plant parts were in the order: roots > leaves > flowers > stems for Cd, Pb and Cu, and leaves > roots > flowers > stems for Mn and Zn. In addition, Page et al. (2006) and Jasion et al. (2013) showed that Zn and Mn transport in plant organs was high and active. These metals are essential micronutrients for plants (Lambers et al. 2008) and readily transported to shoots via xylem (Nworie et al. 2019). However, Jasion et al. (2013) reported that in Tanacetum vulgare not only essential metals such as Mn and Zn but also toxic, non-essential Cd was transported to shoots. Similarly, TF values of > 1 for Mn, Zn but also for toxic Pb were reported for Polygonum arenastrum growing in areas affected by industry (Dambiec et al. 2017). In the present study, active translocation of Ni and Pb (TF > 1) was observed in group 3 where soil was relatively free from pollution. Consequently concentrations of these potentially toxic metals in S. gigantea did not exceed their natural contents in plants given by Markert (1992) and Kabata-Pendias (2011) and they were not likely to pose a toxicity threat. Bielecka and Królak (2019a) reported that Translocation Factors for Pb and Zn in S. canadensis had higher values in the environment with low metal concentrations in soil compared to polluted industrial areas.
Regarding the high BF values for Cd, Cu, Cr, Fe and Ni and limited translocation of these potentially toxic metals from roots to aboveground parts, it can be assumed that S. gigantea could be classified as a metal-tolerant species (Vamerali et al. 2010). Wójcik et al. (2014) also stated that S. gigantea was characterized by lower trace metal concentrations compared to other species (e.g., Anthyllis vulneraria, Echium vulgare or Hieracium piloselloides) that inhabited the same Zn-Pb waste deposits. As an invasive species, S. gigantea cannot be a suitable plant to use in phytoremediation. However, the low potential to translocate Cd, Cu, Cr, Fe and Ni from belowground to aboveground organs in combination with the high BF for these metals suggest that S. gigantea contributes to the reduction in metal mobility and leaching into ground water in areas already inhibited, and it also reduces metal bioavailability for entry into the food chain (Galal and Shehata 2015). On the other hand, the relatively high Zn concentration and its transport to stems and leaves suggest that harvesting the species could contribute to cleaning the soil (phytoextraction) if the biomass were harvested at the time of the maximum Zn contents.
Potential use of Solidago gigantea in bioindication
PCCA ordination (Fig. 4) showed differences between the metal contents in the leaves of S. gigantea from the different study sites and enabled differentiation between sources of pollution. Groups arranged on the factor plane closely correspond to the three categories (groups 1, 2 and 3) related to local sources of pollution and land use type: (1) study sites 1–5 and 12–13, located in industrially polluted sites near the non-ferrous metal refinery and the ironworks, for which factor 1 returned positive scores; (2) study sites 6–11 located in the former metallurgical area, for which factor 2 and factor 1 returned positive and negative scores, respectively; (3) the remaining study sites (14–30), for which factors 1 and 2 returned negative scores, were located mainly in agricultural regions with no large industrial plants, in some cases near roads characterized by various traffic levels. As was previously reported the identified three groups of study sites differed significantly in respect of Cd, Cu, Cr, Mn and Zn concentrations in the leaves of S. gigantea (Kruskal–Wallis ANOVA, p < 0.05) (Fig. 3).
The projection of the variables on the factor plane showed that S. gigantea leaves from industrially polluted sites with positive scores of the first factor were characterized by the highest concentrations of Cd, Cu and Zn. The high content of these metals in the areas affected by the non-ferrous metal refinery were previously recorded by Meinhardt (2016). Solidago gigantea from the former metallurgical area with positive scores of the second factor highly correlated with high Cr and Fe contents. The former ferrochrome steel plant in Siechnice and its slag heap can be the main sources of these metals (Karczewska and Kabała 2010; Meinhardt 2016). Plants from rural regions were correlated more closely with Mn. Manganese concentrations in these plants were higher than in the leaves of S. canadensis growing in a region heavily contaminated with metals (31–61 mg kg−1) (Antonijević et al. 2012). Manganese is used as a micronutrient in fertilizers and as a disinfecting agent so it is a typical element that occurs in urban and agricultural sewage (Reimann and de Caritat 1998; Adriano 2001). The observed relation between metal contents in plants and human activity suggests that S. gigantea could be used as a biomonitor of metal pollution (Kabata-Pendias 2011). What is even more positive, Spearman correlations (p < 0.05) were found between the total and bioavailable concentrations of Cd, Cu and Zn in soil and all of the studied organs of S. gigantea (Table 3). Similar correlations were found between Pb contents in soil (total and bioavailable) and roots of S. gigantea. Such correlations indicate that S. gigantea reflects the cumulative effects of environmental pollution in soil and confirm its potential use in the biomonitoring of elements in general, and Cd, Cu and Zn in particular (Kabata-Pendias 2011). Non-native plants are usually not recommended for monitoring purposes, but S. gigantea is permanently established in European flora (Tokarska-Guzik et al. 2012) and it is noteworthy that, as well as furnishing information about pollutant loads, the species has several other features expected from a passive biomonitor candidate: wide distribution and abundance, ease of identification and sampling, and tolerance to habitat conditions and pollution (Weiss et al. 2003; Zhou et al. 2008). The elevated concentrations in industrial areas should be taken into account when using this species for medicinal purposes to minimize potential human exposure (Mandzhieva et al. 2016; Szymura et al. 2018).
Conclusion
The present study has shown that S. gigantea is able to tolerate elevated levels of potentially toxic metals in soils and to inhabit areas strongly polluted by industry. The species has high BF but low TF values for Cd, Cu, Cr, Fe, Ni. Therefore it can be assumed that S. gigantea easily takes up these metals from soil along with their low mobility to the aboveground organs and could be classified as a metal-tolerant species that adopts the exclusion strategy. The BF values for Cd, Cu, Cr, Pb and Zn were remarkably higher in plants growing in unpolluted areas characterized by low metal concentrations in soil compared to the ones affected by industry, characterized by high metal concentrations, which suggests that S. gigantea growing in polluted areas can reduce the uptake of these potentially toxic metals. These properties can be responsible for the possible advantage of S. gigantea in competition with other species growing in areas affected by human activity and facilitate its invasiveness. Solidago gigantea can reduce the mobility of metals by biding them in roots and its contribution to toxic metal transfer into food chains is relatively low. In addition, it has been shown that the species may be used as a biomonitor of metal pollution, in particular Cd, Cu, Cr and Zn, as concentrations of these elements in leaves of S. gigantea differed between sites differing in land use.
References
Adriano DC (2001) Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York. https://doi.org/10.1007/978-0-387-21510-5
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Antonijević MM, Dimitrijević MD, Milić SM, Nujkić MM (2012) Metal concentrations in the soils and native plants surrounding the old flotation tailings pond of the copper mining and smelting complex bor (Serbia). J Environ Monit 14:866–877. https://doi.org/10.1039/C2EM10803H
Baker AJM (1981) Accumulators and excluders – strategies in the response of plants to heavy metals. J Plant Nut 3(1–4):643–654. https://doi.org/10.1080/01904168109362867
Balicevic R, Ravlić M, Živković T (2015) Allelopathic effect of invasive species giant goldenrod (Solidago gigantea Ait.) on crops and weeds. Herbologia 15(1):19–29. https://doi.org/10.5644/Herb.15.1.03
Benton Jones Jr L (2001) Laboratory guide for conducting soil tests and plant analysis. CRC Press, Boca Raton
Bidar G, Pruvot C, Garçon G, Verdin A, Shirali P, Douay F (2009) Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environ Sci Pollut Res 16(1):42–53. https://doi.org/10.1007/s11356-008-0021-4
Bielecka A (2017) Characteristics of selected properties of soils inhabited by canadian goldenrod. Chem Environ Biotechnol 20:17–20. https://doi.org/10.16926/cebj.2017.20.03 ((in Polish))
Bielecka A, Królak E (2019a) Solidago canadensis as a bioaccumulator and phytoremediator of Pb and Zn. Environ Sci Pollut Res 26:36942–36951. https://doi.org/10.1007/s11356-019-06690-x
Bielecka A, Królak E (2019b) The accumulation of Mn and Cu in the morphological parts of Solidago canadensis under different soil conditions. Peer J 7:e8175. https://doi.org/10.7717/peerj.8175
Biłyk A, Kowal AL (1993) Assessment of chromium contamination hazard of the aquifers of Wrocław. Ochrona Środowiska 1–2:3–6 ((in Polish))
Bobulská L, Cekanová K, Demková L, Obonˇa J, Sarvaš J (2018) Evaluation of the phytoremediation properties of the invasive species Solidago genus. Ann Univ Craiova 23:314–320
Bobulska L, Demkova L, Cerevkova A, Renco M (2019) Invasive goldenrod (Solidago gigantea) influences soil microbial activities in forest and grassland ecosystems in Central Europe. Diversity 11:134. https://doi.org/10.3390/d11080134
Bonanno G, Vymazal J, Cirelli GL (2018) Translocation, accumulation and bioindication of trace elements in wetland plants. Sci Total Environ 631–632:252–261. https://doi.org/10.1016/j.scitotenv.2018.03.039
Chmura M, Dyba P, Kraj P, Peplińska N, Pilorz A, Roman M (2015) Invasion of alien Solidago taxa into urban habitats: a study of selected towns in southern Poland. Chem Didact Ecol Metrol 20(1–2):97–104. https://doi.org/10.1515/cdem-2015-0010
Cuske M, Marcinkiewicz M, Szopka K, Karczewska A, Pora E (2013) The impact of the Oława zinc smelter on the soil environment of the adjacent areas, in the light of the total content of heavy metals in the surface levels of the soils of the city of Oława. Inżynieria Środowiska 29:42–50 ((in Polish))
Czarnowska K (1996) General content of heavy metals in parent rocks as soil geochemical background. Rocz Glebozn 47:43–50 ((in Polish))
Dambiec M, Wojtuń B, Samecka-Cymerman A, Polechońska L, Rudecki A, Kempers AJ (2017) Fluorine and metals in Polygonum arenastrum Bor. from areas influenced by various types of industry. Ecol Indic 82:163–174. https://doi.org/10.1016/j.ecolind.2017.06.053
Eid EM, Shaltout KH, El-Sheikh M, Asaeda T (2012) Seasonal courses of nutrients and heavy metals in water, sediment and above- and below-ground Typha domingensis biomass in Lake Burullus (Egipt): Perspectives for phytoremediation. Flora 207:783–794. https://doi.org/10.1016/j.flora.2012.09.003
Fabiszewski J (2005) Nature of Lower Silesia. KORAB Ltd., Wrocław (in Polish)
Ferreira CSS, Walsh RPD, Ferreira AJD (2018) Degradation in urban areas. Curr Opin Environ Sci Health 5:19–25. https://doi.org/10.1016/j.coesh.2018.04.001
Galal TM, Shehata HS (2015) Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol Indic 48:44–251. https://doi.org/10.1016/j.ecolind.2014.08.013
Gałuszka A, Migaszewski ZM (2011) Geochemical background–an environmental perspective. Miner 42(1):7–17. https://doi.org/10.2478/v10002-011-0002-y
Głowicki B, Otop I, Urban G, Tomczyński K (2005) Climate in: AN ecophysiographic study for the Dolnośląskie Voivodeship. Wrocław, Board of the Lower Silesian Voivodeship, Voivodeship Urban Office in Wrocław (in Polish)
Jasion M, Samecka-Cymerman A, Kolon K, Kempers AJ (2013) Tanacetum vulgare as a bioindicator of trace-metal contamination: a study of a naturally colonized open-pit lignite mine. Arch Environ Contam Toxicol 65:442–448. https://doi.org/10.1007/s00244-013-9922-4
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC Press, Boca Raton
Kalra Y (2019) Handbook of reference methods for plant analysis, 1st edn. CRC Press
Kandziora-Ciupa M, Nadgórska-Socha A, Barczyk G, Ciepał R (2017) Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology 26:966–980. https://doi.org/10.1007/s10646-017-1825-0
Karczewska A, Bortniak M (2008) Chromium and other heavy metals in soils of Wrocław aquifers in the vicinity of the ferrochromic slag heap in Siechnice. Roczn Glebozn - Soil Sci Annu 59(1):106–111 ((in Polish))
Karczewska A, Kabała C (2010) Soils contaminated with heavy metals and arsenic in Lower Silesia - the needs and methods of reclamation. Zesz Nauk UP We Wroc, Rol XCVI 576:59–79 ((in Polish))
Kowalska J, Stryjewska E, Bystrzejewska-Piotrowska G, Lewandowski K, Tobiasz M, Pałdyn J, Golimowski J (2012) Studies of plants useful in the re-cultivation of heavy metals-contaminated wasteland—a new hyperaccumulator of barium? Pol J Environ Stud 21(2):401–405
Kubicz J (2014) Health risk assessment of allotment gardens in Wrocław. Econ Environ 1(48):154–163 ((in Polish))
Lambers H, Chapin FS, Pons TL (2008) Plant physiological ecology, 2nd edn. Springer, New York. https://doi.org/10.1007/978-0-387-78341-3
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Developments in environmental modeling, Amsterdam
Lewicki Z (2014) The environment of Wrocław - Guidebook 2014. Lemitor Ochrona Środowiska Sp. z o.o., Wrocław (in Polish)
Li J, Leng Z, Wu Y, Du Y, Dai Z, Biswas A, Zheng X, Li G, Mahmoud E, Jia H, Du D (2021) Interactions between invasive plants and heavy metal stresses: a review. J Plant Ecol. https://doi.org/10.1093/jpe/rtab100
Liu X, Ouyang W, Shu Y, Tian Y, Feng Y, Zhang T, Chen W (2019) Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ Pollut 254(B):113113. https://doi.org/10.1016/j.envpol.2019.113113
Luo X, Bing H, Luo Z, Wang Y, Jin L (2019) Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: a review. Environ Pollut 255(1):113138. https://doi.org/10.1016/j.envpol.2019.113138
Mandzhieva SS, Minkina TM, Chaplygin V, Motuzova GV, Sushkova SN, Bauer TV, Nevidomskaya DG (2016) Plant contamination by heavy metals in the impact zone of Novocherkassk Power Station in the south of Russia. J Soils Sediments 16:1383–1391. https://doi.org/10.1007/s11368-015-1098-2
Markert B (1992) Presence and significance of naturally occurring chemical elements of the periodic system in the plant organism and consequences for future investigations on inorganic environmental chemistry in ecosystems. Vegetatio 103:1–30. https://doi.org/10.1007/BF00033413
Meinhardt B (2016) Assessment of the degree of soil contamination in the Dolnośląskie Voivodeship based on many years of monitoring studies by WIOŚ Wrocław - 2010–2015. WIOŚ Wrocław (in Polish)
Nowińska K, Kokowska-Pawłowska M, Patrzałek A (2012) Metals in Calamagrostis epigejos and Solidago sp. from reclaimed post-industrial wasteland. Infrastructure and Ecology of Rural Areas. 3(3), Polska Akademia Nauk, Kraków (in Polish)
Nworie OE, Qin J, Lin C (2019) Trace element uptake by herbaceous plants from the soils at a multiple trace element-contaminated site. Toxics 7(3):1–14. https://doi.org/10.3390/toxics7010003
Page V, Weisskopf L, Feller U (2006) Heavy metals in white lupin: uptake, root-to-shoot transfer and redistribution within the plant. New Phytol 171(2):329–341. https://doi.org/10.1111/j.1469-8137.2006.01756.x
Pisula N, Meiners SJ (2010) Allelopathic effects of goldenrod species on turnover in successional communities. Am Midl Nat 163:161–172. https://doi.org/10.1674/0003-0031-163.1.161
Potocki R (2014) Map of threats of the Wrocław District. Poviat Starosty in Wrocław. Team for Crisis Management. Wrocław (in Polish)
Reimann·C, de Caritat P (1998) Chemical Elements in the Environment. Factsheets for the Geochemist and Environmental Scientist. Springer-Verlag Berlin Heidelberg
Sáňka M, Strnad M, Vondra J, Paterson E (1995) Sources of soil and plant contamination in an urban environment and possible assessment methods. Int J Environ an Ch 59(2–4):327–343. https://doi.org/10.1080/03067319508041338
Scharfy D, Eggenschwiler H, Venterink HO, Edwards PJ, Gusewell S (2009) The invasive alien plant species Solidago gigantea alters ecosystem properties across habitats with differing fertility. J Veg Sci 20:1072–1085. https://doi.org/10.1111/j.1654-1103.2009.01105.x
Sołtysiak J, Brej T (2014) Invasion of Fallopia genus plants in urban environment on the example of Wrocław city. Pol J Environ Stud 23:449–458
Sorensen MA, Parker DR, Trumble JT (2009) Effects of pollutant accumulation by the invasive weed saltcedar (Tamarix ramosissima) on the biological control agent Diorhabda elongata (Coleoptera: Chrysomelidae). Environ Pollut 157(2):384–391. https://doi.org/10.1016/j.envpol.2008.10.001
StatSoft Inc (2016) STATISTICA (data analysis software system), version 13
Stefanowicz AM, Stanek M, Woch MW, Kapusta P (2016) The accumulation of elements in plants growing spontaneously on small heaps left by the historical Zn-Pb ore mining. Environ Sci Pollut Res 23:6524–6534. https://doi.org/10.1007/s11356-015-5859-7
Szymura M, Szymura TH (2013) Soil preferences and morphological diversity of goldenrods (Solidago L.) from south-western Poland. Acta Soc Bot Pol 82(2):107–115. https://doi.org/10.5586/asbp.2013.005
Szymura M, Szymura TH (2015) The dynamic of growth and flowering of invasive Solidago species. Steciana 19(3):143–152. https://doi.org/10.12657/steciana.019.016
Szymura M, Szymura TH, Świerszcz S (2016) Do landscape structure and socio-economic variables explain the Solidago invasion? Folia Geobot 51:13–25. https://doi.org/10.1007/s12224-016-9241-4
Szymura M, Gzdęga K, Tokarska-Guzik B (2018) Analysis of the degree of invasiveness of alien species in Poland together with an indication of species that are a significant threat to native flora and fauna and a proposal of strategic actions in terms of the possibility of combating them, and Analysis of the routes of unintentional introduction or spread of invasive alien species along with the development of action plans for priority roads. Species Information Sheet. (in Polish)
Tokarska-Guzik B, Dajdok Z, Zając M, Zając A, Urbisz A, Danielewicz W, Hołdyński C (2012) Plants of foreign origin in Poland, with particular emphasis on invasive species. General Directorate for Environmental Protection. Warszawa (in Polish)
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17. https://doi.org/10.1007/s10311-009-0268-0
Weiss P, Offenthaler I, Ohlinger R, Wimmer J (2003) Higher plants as accumulative bioindicators. In: Markert BA, Breure AM, Zechmeister HG (eds) Bioindicators and biomonitors. Elsevier Science Ltd, Kidlington, UK, pp 465–500
Wójcik M, Sugier P, Siebielec G (2014) Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci Total Environ 487:313–322. https://doi.org/10.1016/j.scitotenv.2014.04.024
Wong CZC, Li X, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142(1):1–16. https://doi.org/10.1016/j.envpol.2005.09.004
Xiang YC, Feng T, Liu BG, Li HG, Chen Y (2010) Growth and accumulation character of heavy metals for Solidago canadensis grown in amended manganese mining tailing. Miner Eng Res 25(1):63–68
Yang RY, Tang JJ, Yang YS, Chen X (2007) Invasive and non-invasive plants differ in response to soil heavy metal lead contamination. Bot Stud 48:453–458
Yang R, Yu G, Tang J, Chen X (2008) Effects of metal lead on growth and mycorrhizae of an invasive plant species (Solidago canadensis L.). J Environ Sci 20(6):739–744. https://doi.org/10.1016/S1001-0742(08)62121-X
Zar J (1999) Biostatistical analysis, 4th edn. Prentice-Hall, New Jersey
Zheljazkov VD, Jeliazkova EA, Kovacheva N, Dzhurmanski A (2008) Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ Exper Bot 64(3):207–216. https://doi.org/10.1016/j.envexpbot.2008.07.003
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150. https://doi.org/10.1016/j.aca.2007.11.018
Acknowledgments
The authors wish to thank all who assisted in conducting this work.
Funding
This work was supported by the National Science Center, Poland [Grant Number 2018/02/X/NZ8/03659].
Author information
Authors and Affiliations
Contributions
MD contributed to conceptualization, methodology, formal analysis, investigation, writing—original draft, funding acquisition; AK contributed to formal analysis, writing—review and editing; LP contributed to investigation, writing—review and editing, visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Editorial responsibility: Tanmoy Karak.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dambiec, M., Klink, A. & Polechońska, L. Concentration and translocation of trace metals in Solidago gigantea in urban areas: a potential bioindicator. Int. J. Environ. Sci. Technol. 19, 11729–11740 (2022). https://doi.org/10.1007/s13762-022-03932-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-03932-3