Abstract
This study re-assess the environmental impacts of the Dexing copper mine (the largest open-pit copper mine in Asia) on the Lean river and its two tributaries (the Dawu river and Jishui river) in the Jiangxi province, China, with particular focus on metal pollution as well as the effectiveness and side-effects of remediation activities. Results show that the Dawu river and its mixing zone with the Lean river were still heavily influenced by acid mine drainage (AMD) and loaded with elevated levels of metals, in particular Mn, Ni, and Al whose concentrations were frequently above the health-based guideline values. Manganese and Ni in the AMD-impacted waters were predicted to occur as free ions or sulfate and carbonate complexes, and thus highly-toxic to living organisms. Although Al in the AMD-impacted waters was predicted to exist largely as colloidal hydroxides with low bioavailability, abundant formation of such nano-sized particles could impair the respiratory and circulatory systems of aquatic macro-invertebrates. The integration and comparison of the results from the current and previous studies show that the concentrations of several metals (Cu, Zn, and Cd) in the Dawu river decreased significantly after 2011–2012, during which several remediation practices were implemented (e.g., AMD neutralization, excavation of contaminated sediments in impounded rivers, and rehabilitation of mine tailings and open-pit slopes). This provides evidence that these remediation practices have effectively limited the dispersion of metals from the mining area. However, AMD neutralization greatly enhanced the release of sulfate, making the mining area an even more important sulfate source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater shortage is one of the greatest global challenges for the twenty-first century (Mekonnen and Hoekstra 2016). Also, increasing water quality deterioration by various organic and inorganic pollutants further increases the pressures on safe freshwater resources worldwide. As a group of non-biodegradable pollutants, potentially toxic metals (hereafter referred to as “metals”) can persist and accumulate in the environment and are thus of great environmental concern. Numerous studies have reported that anthropogenic activities, in particular the development of mining industries, have significantly increased the levels of metals in many aquatic systems (Da Silva et al. 2005; Espana et al. 2005; Balistrieri et al. 2007; Peng et al. 2009; Aleksander-Kwaterczak and Helios-Rybicka 2008; Delgado et al. 2008; Hakkou et al. 2008; Equeenuddin et al. 2010; Thorslund et al. 2012, 2014; Jarsjö et al. 2017; Åhlgren et al. 2020). During the latest decades, a number of remediation strategies/practices (e.g., physico-chemical treatments of mine tailings and associated acidic drainage, land reclamations, and soil detoxification using metal accumulators/hyperaccumulators) have been developed and tested in different mining areas, with overall aims to (i) limit and reduce the release and dispersion of metals from exposed waste dumps/tailings and excavated rocks to recipient water bodies; and (ii) clean up metals and restore ecological systems at contaminated sites (Salt et al. 1995; Mendez and Maier 2008; Khan and Jones 2009; Ali et al. 2013; Wang et al. 2017; Tordoff et al. 2000). However, the effectiveness and potential side effects of these remediation strategies/practices are as yet unclear in many areas and thus, require more detailed monitoring and assessments over long time scales. For instance, acid neutralization of mine tailings and associated acidic drainage (a common remediation practice in many mining areas) might greatly enhance the mobilization and transport of sulfate, due to rapid pH-driven removal of Fe3+, Al3+, Ca2+, and other cations that would otherwise precipitate as a range of (oxyhydro-)sulfate minerals (e.g., schwertmannite, jarosite, jurbanite, alunite, and gypsum) under natural acidic conditions (Domènech et al. 2002; Burton et al. 2006; Yu et al. 2014).
It is well-known that aqueous speciation of metals, i.e., in which physical and chemical forms they occur, controls their toxicity, bioavailability, and mobility in the natural environment (Fytianos 2001; Thorslund et al. 2014, 2016). For instance, free ions and inorganic complexes are generally considered to be the most mobile, bioavailable, and toxic forms for most of the metals (Nystrand and Österholm 2013). Thus, knowledge of the aqueous speciation of metals is not only a prerequisite for an accurate assessment of their toxicological effects/risks, but also essential in identifying the key hydrological and geochemical processes controlling their mobilization, transport, and fate in the environment (Nystrand and Österholm 2013; Thorslund et al. 2014; Nystrand et al. 2016; Yu et al. 2019). The latter information will also allow us to predict how the pollution and chemical status of metals will evolve with time in response to possible environmental changes (Wällstedt et al. 2010; Sjöstedt et al. 2013).
The Dexing mining area, located in the northeastern part of Jiangxi province, China, is famous for its abundant non-ferrous metal sulfide resources (He et al. 1997; Wu et al. 2014). There are two large-sized mines in this area, the Yinshan Lead–Zinc mine and the Dexing Copper mine (the largest open-pit copper mine in Asia), which have been exploited for more than 50 years (Yang 2011; Zhou et al. 2018). These two mines are located in the upstream section of the Lean river (approximately 279 km in length), which is an important water source for millions of inhabitants in its catchment (9616 km2) (Chen et al. 2016). The pollution of the Lean river water by acid mine drainage (AMD) from the Dexing mining area can be traced back to more than 30 years ago (the first documented sampling was conducted in 1987). Since then, a large number of studies have been carried out in this area, focusing on metal pollution and associated ecological risks in riverine waters (He et al. 1998; Yanguo et al. 2004; Xiao et al. 2009; Sjöstedt et al. 2013; Tao et al. 2014; Liu et al. 2020), riverine sediments (He et al. 1997, 1998; Liu et al. 1999, 2020; Yanguo et al. 2004; Xiao et al. 2009, 2011; Tao et al. 2014; Chen et al. 2016; Liang et al. 2019), floodplains (Xiao et al. 2011; Liang et al. 2019), soils (Yanguo et al. 2004; Teng et al. 2010; Guo et al. 2011; Liu et al. 2013; Wu et al. 2014; Yu et al. 2016; Zhou et al. 2018; Lin et al. 2019; Hu et al. 2019) and crops/vegetables (Yu et al. 2016; Zhou et al. 2018). The most recent systematic study on the river system in the mining area was conducted in 2005 (Xiao et al. 2009). This study showed that, although the ore production of the two mines increased year by year, the riverine and sediment-bound metal loads in the Lean river only slightly increased or even started to decline as compared to those reported about 15 years ago, presumably due to technical improvements in utilizing ores of low-grades (Xiao et al. 2009). This study also reported that the Dawu river, a tributary of the Lean river, running through the copper mine was still heavily affected by AMD originating from the open pits and waste rocks in its upper reach. Since 2011–2013, a few remediation projects were initiated and implemented to mitigate the negative impacts of the mining activities in the area, such as neutralization of AMD, regular excavation of contaminated sediments in impounded rivers, and rehabilitation of contaminated sites (IPEN 2015; Li 2018; Wu 2018). However, there is no systematic study to investigate the current levels of metals as well as their speciation and hydrogeochemical controls in the river system within the mining area after the implementation of these remediation projects.
The main aims of this study were to evaluate (i) current levels, speciation, and hydro-geochemical controls of metals in the river system of the mining area; and (ii) whether and to what extent the remediation practices have mitigated negative impacts of the mining activities and enhanced sulfate leaching in the area.
Materials and methods
Study area
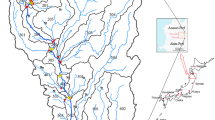
The study area (the Dexing mining area) is located in the northeastern part of Jiangxi province, China (Fig. 1). The Lean river runs through the area from north-east to south-west and eventually flows into the Poyang Lake, the largest freshwater lake in China. The climate of the study area is strongly influenced by the eastern Asia summer monsoon, with an average annual temperature of approximately 19 °C, and annual mean precipitation of around 1800 mm (Pei et al. 2019). The rainy season starts in April and ends in September. The main soil types in the area are paddy soil, yellow soil, and red soil (Zhou et al. 2018). Paddy soil is mainly distributed along rivers and streams, while the latter two soils mainly occur in hilly areas (Teng et al. 2010; Zhou et al. 2018).
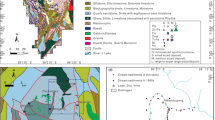
Geologically, the study area is located close to the tectonic suture zone (Jiangshan-Shaoxing suture zone) between the Yangtze and Cathaysia blocks and mainly underlain by Neoproterozoic metamorphic rocks (mainly phyllite and slate) (Fig. 2). To the north-eastern corner of the study area, the Dexing Cu-bearing porphyries intruded the Neoproterozoic metamorphic rocks during the early Middle Jurassic (Zhou et al. 2012). The porphyries consist mainly of granodiorite-porphyry with minor quartz monzodiorite-porphyry (Zhou et al. 2012). To the south of the area, a volcanic-sedimentary sequence lies along the northern margin of the Cathaysia block. The sedimentary sequence was mainly deposited during the early Cretaceous and has a total thickness of 8000–10,000 m (Hou et al. 2013).
Sketch showing general geology of the study area and surroundings (modified after Jiang et al. 2011)
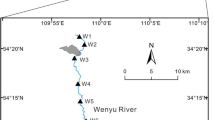
As two major branches of the upper reach of the Lean river, the Jishui river (39 km long, 19.3 m/s of current velocity) and the Dawu river (14 km long, 0.3–1.5 m/s of current velocity) runs through the Yinshan Lead–Zinc mine and the Dexing Copper mine, respectively (Liu et al. 2003). Mining operations of the Yanshan Lead–Zinc mine were conducted mainly in opencast, but also extensively underground, resulting in over 20 km of interconnected tunnels (Hu et al. 2019). Annually, it produces around 0.7 million tons of crude ore, in which galena, chalcopyrite, sphalerite, and pyrite are the dominant sulfide minerals (Xiao et al. 2011; Yang 2011). The Dexing copper Mine has an annual production of over 30 million tons of crude ore (Wu et al. 2009; Xiao et al. 2011). The ore minerals include pyrite, chalcopyrite, molybdenite, shaperite, galena, native gold, and magnetite (Pan et al. 2017). The exploitation of the mine has produced hundreds of millions of ore tailings (Wu et al. 2009), which were reported to contain several sulfide minerals, including pyrite (3.5%), chalcopyrite, sphalerite, and galena (Tian and Li 2000). To take advantage of natural slopes for maintaining the stability of pile and for facilitating the drainage of water, ore tailings were transported from open-pit to dump sites along the upstream valley of the Dawu river (Mishra et al. 2008; Wu et al. 2009). The dumped tailings are estimated to cover a total area of approximately 7.6 km2 (Wu et al. 2009). After reacting with air and rainwater via microbial activity (e.g., Fe-oxidizing chemolithoautotrophs), the sulfide minerals in the tailings released abundant reddish AMD of very low pH (2.5–2.6) and high sulfate concentrations (> 38,000 mg/L) (Pei et al. 2019). According to a recent study by Wu et al. (2020), the precipitation in the study area is weakly acidic (pH = 6.2) and contains moderate levels of acidifying compounds (SO42− = 1.89 mg/L and NO3− = 0.31 mg/L), and thus should have contributed to the generation of AMD in the mining area. Shallow groundwater in the study area is neutral to slightly alkaline (pH = 7.1–7.3), and belongs to a calcium-magnesium-sulfate water type with total hardness of 3–46 mg/L (Jiangxi Provincial Bureau of Geology 1980).
Water sampling and analyses
Surface water samples were collected from a total of 10 sites along the mainstream of the Lean river (LA-1 to LA-4), the Dawu river (DW-1, DW-2, DW-3, and DW-3mix), and the Jishui river (JS-1 and JS-2) on two occasions, one in October 2017 (dry season) and one in August 2018 (rainy season) (Figs. 1 and 3, Table 1). In the field, temperature, pH, electrical conductivity (EC), and redox potential were measured in situ, directly in river water at each site, using pre-calibrated portable Hach meters (HQ30d, Hach company, Loveland, Colorado, USA). Thereafter, one sample of surface water was collected by slowly submerging an acid-washed polyethylene bottle (500 mL) just below the water surface. The bottled water was then transferred into two 50 mL Falcon tubes, one for determining the concentrations of truly-dissolved plus colloidal fractions (hereafter collectively referred to as “dissolved” fraction) of cations and metals (filtered through 0.45 µm membranes before being acidified to pH < 2 with ultrapure HNO3), and one for measuring the concentrations of “total acid-available” fraction of cations and metals (immediately acidified to pH < 2 with ultrapure HNO3). The “total acid-available” fraction consists of the “dissolved” fraction plus part of the particulate phase that can easily be dissolved under acidic conditions. In the 2018 campaign, two additional filtered (0.45 µm) water samples were obtained from each site, one for dissolved organic carbon (DOC) analysis and one for alkalinity and anion analysis. All the water samples were stored in darkness at 4 ℃ prior to further analyses.
The acidified unfiltered and filtered water samples were analyzed in randomized order for 16 elements (K, Na, Ca, Mg, Fe, Al, Si, Mn, Sr, Cu, Zn, Ni, Co, Cd, Pb, and U) at the Analytical Laboratory of the Beijing Research Institute of Uranium Geology by inductively coupled plasma (ICP)- mass spectrometry (MS) or atomic emission spectroscopy (AES) using standard methods (DZ/T 0064.80-1993 and GB/T 5750.6/1.4-2006). The precision calculated based on measurements of blind duplicate samples was better than 5% (RSD) for the determined elements, except Fe whose precision was approximately 14% (RSD). Alkalinity was determined using a standard titration method (Wei et al. 2002). Concentrations of DOC were analyzed using a TOC analyzer (Aurora 1030 W TOC Analyzer, OI Scientific), while the concentrations of sulfate and chloride using ion chromatography (Dionex ICS-1100), following Chinese national standard methods (HJ 84-2016 and GB/T 5750.5-2006).
Geochemical modeling
The geochemical equilibrium program Visual MINTEQ version 3.1. (Gustafsson 2014) was used to predict the speciation of “dissolved” metals in the filtered waters collected in 2018. Input parameters included pH, temperature, alkalinity (HCO3−) and concentrations of major cations (Ca2+, K+, Na+, and Mg2+), anions (Cl−, F−, and SO42−), DOC, Al, Fe, Mn, Si, Cu, Co, Zn, Ni, Cd, Pb, and U.
For modeling the redox speciation of Fe and Mn, the redox couples Fe2+/Fe3 + and Mn2+/Mn3+ were added. The NICA-Donnan model implemented in Visual MINTEQ was used to simulate the formation of metal-dissolved organic matter (DOM) complexes. It was assumed that the ratio of active DOM to DOC was 1.65 and that 82.5% of the active DOM was fulvic acid and the remaining 17.5% inactive with respect to proton and metal binding (Sjöstedt et al. 2013).
In the calculations, the Al, Fe, Mn and Ca in the water samples were allowed to precipitate when the solubility products for Al(OH)3 (soil) (log*Ks = 8.29 at 25 °C), ferrihydrite (aged) (log*Ks = 2.69 at 25 °C), birnessite (log*Ks = 18.091 at 25 °C), and calcite (log*Ks = − 8.48 at 25 °C) were exceeded. The rationales for including these possible solid phases were: (i) ferrihydrite- and gibbsite-like phases are important colloids in surface waters, (ii) ferrihydrite, birnessite, and gibbsite possess large reactive surfaces that are negatively charged under neutral and alkaline conditions, and thus have the greatest potential to act as inorganic sorbents for metals, and (iii) Fe, Al, Mn, and Ca ions can compete with metals for ligand-binding sites on DOM.
The binding of metals to gibbsite and ferrihydrite were modeled if these phases were predicted to occur in the water samples. Ferrihydrite and gibbsite were assumed to have a specific surface area of 600 m2 g−1 and 32 m2 g−1, respectively. Sorption to gibbsite was simulated with the diffuse layer model (DLM) (Karamalidis and Dzombak 2010) and to ferrihydrite with the CD-MUSIC model parameterized for this mineral (Tiberg et al. 2013).
Results and discussion
General water chemistry
The surface waters from the Dawu river (sites DW-1, DW-2, and DW-3) and its mixing zone with the Lean river (site DW-3mix) exhibited distinctly different physicochemical characteristics as compared to those from the other sites. These waters were characterized by relatively low pH (5.9–7.4, as compared to the other sites with pH values of 7.4–8.7) and alkalinity (8.63–25.0 m/L HCO3−), but high EC (754–3220 µS/cm) and “dissolved” concentrations of sulfate and major cations (Ca, Mg, and Na) (Table 1). The “dissolved” concentrations of sulfate in these waters were exceptionally high, varying from 389 mg/L (site DW-3mix) to 2862 mg/L (site DW-1), which are 107–784 times greater than the concentration at site LA-1 located in the lower part of the upper reach of the Lean river where the water chemistry is not significantly affected by human activities (He et al. 1998; Xiao et al. 2009). Although the concentrations of DOC and chloride did not vary significantly among the sampling sites, it appears that the Dawu river (in particular its upper reach) was overall loaded with higher levels of DOC (2.0–5.6 mg/L) and chloride (2.4–4.2 mg/L) than the other two rivers (Table 1). The water from the middle reach of the Dawu river (site DW-2) was weakly alkaline, due to the input of alkaline effluents from a dressing plant and its associated tailing pond, located approximately 10 km upstream from this sampling site (Pan et al. 2017).
Although the waters from the Jishui river and the downstream part of the Lean river (after site DW-3mix, that is, LA-2 to LA-4) had similar physicochemical properties as site LA-1, such as weakly- to moderately alkaline conditions (pH = 7.4–8.7) and varying but overall stable values of EC and concentrations of “dissolved” major cations, the “dissolved” concentrations of sulfate in these waters were strongly elevated (Table 1). In addition, hydrological conditions (e.g., water flow) had a strong impact on the water chemistry of the three rivers. For instance, the values of EC as well as the “dissolved” concentrations of major cations in the surface waters collected in the dry season (October 2017), were significantly higher than in the waters sampled from the same sites in the rainy season (August 2018) (Table 1).
“Dissolved” and “total acid-available” concentrations of metals
In the heavily metal-loaded Dawu river, the concentrations of metals both in the “dissolved” and “total acid-available” fractions were very high in the upper reaches (DW-1) and decreased rapidly, and in most cases steadily, along the flow path toward the outlet of the river (Fig. 4, Table 1). The only exception was Pb, for which the concentrations of both fractions did not vary much along this river. During the 2017 low-flow campaign when water was sampled only from site DW-3, the concentrations at this site were, as compared to the 2018 high-flow campaign, higher for Fe, Mn, Ni, U, Al, Co, and Pb, similar for Zn and Cu, and lower for Cd (Fig. 4, Table 1). A characteristic feature for this river was that several metals (Mn, Zn, Ni, Co, and Cd) occurred in similar concentrations in the “dissolved” and “total acid-available” fractions, showing that the loads of these metals in this river were composed entirely of truly dissolved and colloidal phases.
For the Jishui river, the spatial variation in metal concentrations was the opposite to that in the Dawu river with lower concentrations (except for U) in the upper reaches (JS-1) as compared to the lower reaches (JS-2). As a consequence, in the lower reaches of the Jishui river the concentrations of two metals (Zn and Cd) were consistently higher than those in the lower reaches of the metal-rich Dawu river. Another hydrochemical feature for the Jishui river was higher ratios of “total acid-available” to “dissolved” concentrations in the upper than the lower reaches for most of the metals (Fe, Mn, Zn, Ni, Al, Co, Pb, and Cd). As a consequence, not only did the concentrations, but also the proportions of the truly dissolved plus colloidal fractions, of these metals increase along this river.
In the Lean river, only Cd showed a consistent increase in concentrations down river, reflecting the inflow of the Cd-rich Dawu river between LA-1 and LA-2 and the even Cd-richer Jishui river between LA-2 and LA-3 (Fig. 4, Table 1). For other metals, there was a consistent down river increase in the “dissolved” concentrations only (U, Cu, Co). For all metals except Cd, there was a substantial increase in “total acid-available” concentrations between LA-1 and LA-2 but thereafter no increase. Other characteristic features of this river were that, during the low-water flow campaign, the concentrations at LA-3 (downstream of the studied tributaries) were higher than, or similar to those, during the high-flow campaign, and that for several metals the concentrations were much larger in the “total acid-available” than the “dissolved” fraction (in particular Fe, Mn, Al, Co, and Pb).
Speciation of “dissolved” metals
Visual Minteq modeling predicted that “dissolved” Fe and Al occurred abundantly as colloidal hydroxides (ferrihydrite: 22–100% and gibbsite: 40–100%) at most of the sampling sites (Table 2). The remaining Fe in the Lean river and the Jishui river was predicted to occur as Fe(III)-organic complexes, and in the upper and lower reaches of the Dawu river also as free inorganic ion, sulfate, and carbonate complexes, while the remaining Al largely as free inorganic ion at all sites. “Dissolved” Mn was, on the other hand, predicted to be free from colloidal hydroxide (birnessite), occurring predominantly as free inorganic ion (56–93%), and to a lesser but significant extent to as sulfate and carbonate complexes. “Dissolved” Cu and Pb were predicted to be almost entirely bound to DOC (> 92%) in the Lean river and the Jishui river, and in the Dawu river additionally present as free inorganic ions, sulfate, and carbonate complexes. The “dissolved” fractions of Zn, Ni, Co, and Cd were modeled to be dominated by free ions, sulfate, and carbonate complexes in the Dawu river, and additionally by soluble organic complexes in the Lean river and the Jishui river. “Dissolved” U was modeled to be almost completely bound to carbonate, except for sites LA-1 and DW-1 where large proportions (22–71%) of U(VI)-organic complexes were also predicted. Our modeling simulations also showed that basically no metal was sorbed by colloidal ferrihydrite and gibbsite fractions, except for Zn which was sorbed by gibbsite to a very limited extent (< 1%).
Metal contamination and dispersion in the mining area: current status and hydro-geochemical controls
Xiao et al. (2009) carried out a systematic study on the chemistry of major ions and metals in the Lean river and its major tributaries in 2005, and found that the Dawu river, in particular its upper reach (with pH and EC reaching 2.8 and 6170 µs/cm, respectively), was severely impacted by AMD. Similarly, the surface waters in the Dawu river (in particular its upper reach) during our sampling campaigns also displayed characteristics that are typical for AMD, such as low pH (down to 5.9), but high EC (up to 3220 µs/cm) as well as “dissolved” concentrations of sulfate, cations, and metals (Table 1 and Fig. 4). This indicates that, after 12–13 years, the water chemistry of the Dawu river was influenced to a lesser but still significant extent by AMD from the mining pits and tailings of the Dexin copper mine. The Dawu river, in particular its upper reach, was strongly polluted by Al and Mn, whose “dissolved” and/or “total acid-available” concentrations greatly exceeded the World Health Organization and Chinese guideline values for drinking water on both sampling occasions (Tables 1, 3, and Fig. 4). The “dissolved” and “total acid-available” concentrations of Ni in the Dawu river also frequently exceeded the guideline values, depending on the sampling locations or seasons (Tables 1, 3, and Fig. 4). In particular, the “dissolved” fractions of Mn and Ni were predicted to be dominated by free inorganic ions or sulfate complexes (Table 2), with high bioavailability and thus toxicological effects. Chronic exposure or intake of these two metals could cause a variety of adverse effects on aquatic macro-organisms (e.g., fishes) and humans (O’Neal and Zheng 2015; Genchi et al. 2020). Although Al in the river was predicted to exist largely as Al hydroxides with low bioavailability (Table 2), it has been shown that the formation of abundant Al hydroxide colloids could impair the respiratory and circulatory systems of fishes and other aquatic macro-invertebrates by clogging their gills (Gensemer and Playle 1999; Bjerknes et al. 2003). Therefore, the environmental fate and potential toxicological effects of these metals carried by the Dawu river are the major concern in the mining area.
The water chemistry of the Jishui river was also impacted by the lead–zinc mine located in its middle reach (Fig. 1), as reflected by a strong increase in the concentrations of both “dissolved” and “total acid-available” fractions of most of the metals (except U) in the middle reach of the river relative to its upper reaches (Table 1 and Fig. 4). However, the concentrations of these two fractions for most of the metals were low, except for Al, Mn, and Pb whose “total acid-available” concentrations were slightly higher than the WHO and Chinese guideline values on one occasion (Fig. 4 and Table 3). Therefore, the water quality of the Jishui river was not heavily impacted by the mining activities in its catchment, as reported also by Xiao et al. (2009).
In line with the findings of previous studies (He et al. 1997, 1998; Liu et al. 2003; Xiao et al. 2009), the input of the acidic and metal-rich water from the Dawu river had a strong impact on the water quality of the Lean river (Table 1 and Fig. 4). In particular, the “dissolved” and “total acid-available” concentrations of Al, Mn, and Ni in the waters from the confluence point (DW-3mix) of these two rivers as well as its downstream site (LA-2 and LA-3) were occasionally very high and exceeded the WHO and/or Chinese guideline values (Fig. 4 and Table 3). Although “dissolved” fractions strongly dominate the total riverine metal loads in the Dawu river, abundant particulate fractions (> 0.45 µm) were found for most of the metals (in particular Fe, Al, and Mn) in the waters at its mixing site (DW-3mix) with the Lean river (Fig. 4). This contrasting feature suggests that, once mixing with the alkaline water in the Lean river, large fractions of the “dissolved” metal loads carried by the Dawu river were quickly transformed to particulate fractions (> 0.45 µm), due to pH-induced coagulation, (co-)precipitation and sorption processes as found for neutralization of acidic metal-rich waters elsewhere (Åström et al. 2012; Nystrand et al. 2016).
The particulate metal fractions formed at site DW-3mix are expected to quickly settle down to the bottom of the Lean river downstream, as supported by the facts that (i) the “total acid-available” fractions for most of the metals (e.g., Fe, Al, Cu, Co, Cd, Pb, Ni, and U) displayed a stronger downstream decrease along the Lean river (from site LA-2 to LA-4) as compared to their “dissolved” fractions (Fig. 4); and (ii) strongly elevated levels of metals (e.g., Cu, Zn, and Pb) in sediments at the confluence point of the Dawu and Lean rivers as well as the downstream sites as reported previously (He et al. 1998; Liu et al. 2003; Xiao et al. 2009, 2011; Liang et al. 2019). These features suggest that the formation and settling of particulate phases formed at the confluence zone of the Dawu river and Lean river control the dispersion and attenuation of the metal loads from the Dawu river, the only AMD-polluted river in the mining area. It has been shown that organic complexation could greatly enhance the mobility and transport of metals, especially under alkaline conditions (Weng et al. 2002; Li et al. 2013). Taking into account this fact as well as large fractions of DOM-bound metals in the Lean river predicted by our modeling, seasonal variability of DOM input from the catchment is also expected to strongly impact the transport and spreading of the metals from the Dawu river.
Effectiveness and side effects of remediation practices
In the past decades, numerous research and efforts have been made to develop efficient and cost-effective technologies for minimizing the ecological impacts of AMD and associated mine tailings. As reviewed and discussed by Byrne et al. (2012), RoyChowdhury et al. (2015), and Park et al. (2019), the overall aims of these technologies are to (i) inhibit AMD generation processes via physical, chemical, and biological treatment/stabilization of mining tailings (so-called source control technologies); or (ii) immobilize and clean up toxic metals in already produced AMD. Common source control technologies include mixing tailings with benign material (e.g., limestone) or alkaline amendments (e.g., lime), construction of oxygen/water barriers, utilization of bactericides, and phytostabilization (via establishing a vegetative cover on tailing), while the latter involves mainly chemical neutralization of AMD, construction of wetlands and anaerobic bioreactors/drains, and phytoextraction (through planting and harvesting hyper-accumulators).
Metal pollution and potential ecological risks in the Dexing mining area also caught great attention of government authorities at both municipal and provincial levels. According to a report by the International Pollutants Elimination Network (IPEN 2015), the environmental protection agency of the Jiangxi Province, local authorities of Dexing city, and the Dexing copper mine jointly initiated several pioneer projects for controlling pollution sources and phasing out old ore-processing technologies in 2011. In 2013, the Province further invested a few new projects focusing on the treatments of AMD from mine tailings (e.g., by neutralizing with the alkaline effluents from dressing factories) and restoration of open-pit slopes, mine tailings, and contaminated soils in the mining area (IPEN 2015; Li 2018; Wu 2018), as shown in Fig. 5a–d. During our sampling campaigns, we found several dams in the upper reach of the Dawu river (Fig. 5e, f). The sediments accumulated in the impounded river were regularly excavated by the copper mine (personal communications with the local inhabitants). The strong predominance of “dissolved” metal fractions throughout the Dawu river, as found in this study (Table 1, and Fig. 4), indicates that these dams have effectively lowered water velocity and flow, providing sufficient time for particulate phases to settle down before being transported downstream. During the last two decades, the concentrations of “dissolved” Cu, Zn, and Cd in the upper reach of the Dawu river have declined dramatically with time, especially between 2011 and 2012 (Fig. 6). A similar trend was not evident in the middle and lower reach of the river, despite that the “dissolved” concentrations of Cu, Zn, and Cd were occasionally very low in 2004 (Fig. 6). These temporal trends, in combination with limited AMD components in recent Dawu river waters as found by this study, provide strong evidence that the water quality of the Dawu river has significantly improved, in particular after 2011–2012 during which several remediation projects were implemented.

taken from Li. (2018); b treatment of acidic drainage from rehabilitated mine tailings; c and d rehabilitation of mine tailings; e and f sediment retention dams in the upper reach of the Dawu river
Examples of remediation practices in the Dexing mine area: a rehabilitation of open-pit slopes (the insert subfigure shows the original open-pit slopes before rehabilitation, the photos were
Boxplots showing the temporal variations in “dissolved” concentrations of Cu, Zn, and Cd in surface waters sampled from the upper, middle, and lower reaches of the Dawu river. The numbers of water samples are given in the parentheses. Data are gathered from Xue et al. (2012), Xue, (2013), and Yi, (2019). Note, the sampling sites are not the same as ours, and in most of the cases, surface waters were collected from several sites within the upper, middle, and lower reach of the river
The concentrations of sulfate in the Dawu river waters sampled by this study were unexpectedly high (724–2862 mg/L) and increased by two orders of magnitude in comparison with the waters sampled in 2005 by Xiao et al. (2009) (126.8 ± 132.6 mg/L). In contrast, sulfate concentrations in our water samples from the Jishui river (14.2–55.2 mg/L) were within the same range as those reported by Xiao et al. (2009) (51.7 ± 6.0 mg/L). Sulfate is the major component in AMD and is commonly precipitated together with Fe, Al, and Ca as (oxyhydro-)sulfate minerals, such as schwertmannite, jarosite, jurbanite, alunite, and gypsum, in AMD-dominated and other similar acidic sulfate-rich environments (Domènech et al. 2002; Burton et al. 2006; Yu et al. 2014). As a common remediation practice in the catchment of the Dawu river, AMD originating from open pits and tailings was impounded and neutralized by the alkaline effluents from local dressing factories (Fig. 5b). These processes can strongly increase the pH of the treated AMD, favoring the precipitation of hydroxide phases (e.g., Fe and Al hydroxides). The removal of Fe, Al, and other cations as hydroxide phases, which would otherwise precipitate sulfate via forming (oxyhydroxy-)sulfates minerals, favors preferential accumulation of sulfate in the treated AMD. Therefore, the treatments of the AMD from the copper mine have significantly enhanced the mobilization and export of sulfate from the mining area to the Dawu river, the Lean river, and ultimately to the Poyang lake. Also, since sulfate can form strong complexes with metals in the studied rivers (Table 2), the enhancement of sulfate mobilization should have contributed to the mobilization and transport of the metals from the Dawu river.
Conclusion
This study focuses on spatio-temporal characteristics, physicochemical status, hydrological controls, and ecological risks of metals in the upstream section of the Lean river and its two tributaries (the Dawu river and the Jishui river), previously found to be heavily contaminated by acid mine drainage (AMD) from two large-sized mines (the Dexing copper mine and the Yinshan Lead–Zinc mine) within the Dexing mining area in south-eastern China. The main findings are:
-
The Dawu river running through the Dexing copper mine (the largest open-pit copper mine in Asia) was still the most AMD-contaminated river in the mining area, as reported by previous studies conducted more than 12 years ago.
-
The concentrations of Mn, Ni, and Al in the Dawu river and the downstream sites along the Lean river frequently exceeded international and national health risk-based guideline values. In particular, the former two metals were predicted to occur largely in highly toxic forms (as free ions and inorganic complexes), and thus require particular attention.
-
The integration and comparison of the results from this and previous studies indicated that the environmental situation in the mining area has significantly improved during the last decades, due to the improvements of ore-processing technologies and implementation of different remediation practices in the mining area.
-
The levels of sulfate in the Dawu river and its downstream sites along the Lean river strongly increased in comparison with those measured by a previous study in 2005. This unexpected feature most likely reflects preferential release of sulfate during the neutralization of AMD. Since wetlands and anaerobic bioreactors/drains are efficient in fixing sulfate as sulfide minerals, they warrant being tested and implemented as additional remediation methods in the mining area and elsewhere where enhanced release of sulfate was also observed for treated mine tailings and/or AMD.
References
Åhlgren K, Sjöberg V, Grawunder A, Allard B, Bäckström M (2020) Chemistry of acidic and neutralized alum shale pit lakes 50 years after mine closure, Kvarntorp, Sweden. Mine Water Environ 39:481–497. https://doi.org/10.1007/s10230-020-00665-y
Aleksander-Kwaterczak U, Helios-Rybicka E (2008) Contaminated sediments as a potential source of Zn, Pb, and Cd for a river system in the historical metalliferous ore mining and smelting industry area of South Poland. J Soils Sediments 9(1):13–22. https://doi.org/10.1007/s11368-008-0051-z
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7):869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Åström ME, Österholm P, Gustafsson JP, Nystrand M, Peltola P, Nordmyr L, Boman A (2012) Attenuation of rare earth elements in a boreal estuary. Geochim Cosmochim Acta 96:105–119. https://doi.org/10.1016/j.gca.2012.08.004
Balistrieri LS, Seal RR, Piatak NM, Paul B (2007) Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl Geochem 22(5):930–952. https://doi.org/10.1016/j.apgeochem.2007.02.005
Bjerknes V, Fyllingen I, Holtet L, Teien HC, Rosseland BO, Kroglund F (2003) Aluminium in acidic river water causes mortality of farmed Atlantic Salmon (Salmo salar L.) in Norwegian fjords. Mar Chem 83(3–4):169–174. https://doi.org/10.1016/S0304-4203(03)00110-5
Burton ED, Bush RT, Sullivan LA (2006) Sedimentary iron geochemistry in acidic waterways associated with coastal lowland acid sulfate soils. Geochim Cosmochim Acta 70(22):5455–5468. https://doi.org/10.1016/j.gca.2006.08.016
Byrne P, Wood P, Reid I (2012) The impairment of river systems by metal mine contamination: a review including remediation options. Crit Rev Environ Sci Technol 42(19):2017–2077. https://doi.org/10.1080/10643389.2011.574103
Chen H, Chen R, Teng Y, Wu J (2016) Contamination characteristics, ecological risk and source identification of trace metals in sediments of the Le’an River (China). Ecotoxicol Environ Saf 125:85–92. https://doi.org/10.1016/j.ecoenv.2015.11.042
Da Silva EF, Fonseca EC, Matos JX, Patinha C, Reis P, Santos Oliveira JM (2005) The effect of unconfined mine tailings on the geochemistry of soils, sediments and surface waters of the lousal area (Iberian Pyrite Belt, Southern Portugal). Land Degrad Dev 16(2):213–228. https://doi.org/10.1002/ldr.659
Delgado J, Sarmiento AM, Condesso de Melo MT, Nieto JM (2008) Environmental Impact of mining activities in the southern sector of the Guadiana Basin (SW of the Iberian Peninsula). Water Air Soil Pollut 199(1–4):323–341. https://doi.org/10.1007/s11270-008-9882-x
Domènech C, Ayora C, De Pablo J (2002) Sludge weathering and mobility of contaminants in soil affected by the Aznalcollar tailing dam spill (SW Spain). Chem Geol 190(1–4):355–370. https://doi.org/10.1016/S0009-2541(02)00125-0
Equeenuddin SM, Tripathy S, Sahoo PK, Panigrahi MK (2010) Hydrogeochemical characteristics of acid mine drainage and water pollution at Makum Coalfield, India. J Geochem Explor 105(3):75–82. https://doi.org/10.1016/j.gexplo.2010.04.006
Espana JS, Pamo EL, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20(7):1320–1356. https://doi.org/10.1016/j.apgeochem.2005.01.011
Fytianos K (2001) Speciation analysis of heavy metals in natural waters: a review. J AOAC Int 84(6):1763–1769. https://doi.org/10.1093/jaoac/84.6.1763
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: human health and environmental toxicology. Int J Environ Res 17(3):679. https://doi.org/10.3390/ijerph17030679
Gensemer RW, Playle RC (1999) The bioavailability and toxicity of aluminum in aquatic environments. Crit Rev Environ Sci Technol 29(4):315–450. https://doi.org/10.1080/10643389991259245
Guo G, Yuan T, Wang W, Li D, Cheng J, Gao Y, Zhou P (2011) Bioavailability, mobility, and toxicity of Cu in soils around the Dexing Cu mine in China. Environ Geochem Health 33(2):217–224. https://doi.org/10.1007/s10653-010-9334-6
Gustafsson JP (2014) Visual MINTEQ 3.1. Stockholm: KTH, Deptartment of Land and Water Resources Engineering
Hakkou R, Benzaazoua M, Bussière B (2008) Acid mine drainage at the abandoned Kettara Mine (Morocco): 2. Mine waste geochemical behavior. Mine Water Environ 27(3):160–170. https://doi.org/10.1007/s10230-008-0035-7
He M, Wang Z, Tang H (1997) Spatial and temporal patterns of acidity and heavy metals in predicting the potential for ecological impact on the Le An river polluted by acid mine drainage. Sci Total Environ 206(1):67–77. https://doi.org/10.1016/S0048-9697(97)00217-9
He M, Wang Z, Tang H (1998) The chemical, toxicological and ecological studies in assessing the heavy metal pollution in Le An River, China. Water Res 32:510–518. https://doi.org/10.1016/S0043-1354(97)00229-7
Hou Z, Pan X, Li Q, Yang Z, Song Y (2013) The giant Dexing porphyry Cu–Mo–Au deposit in east China: product of melting of juvenile lower crust in an intracontinental setting. Miner Depos 48(8):1019–1045. https://doi.org/10.1007/s00126-013-0472-5
Hu J, Lin B, Yuan M, Lao Z, Wu K, Zeng Y, Liang Z, Li H, Li Y, Zhu D, Liu J, Fan H (2019) Trace metal pollution and ecological risk assessment in agricultural soil in Dexing Pb/Zn mining area, China. Environ Geochem Health 41(2):967–980. https://doi.org/10.1007/s10653-018-0193-x
IPEN (2015) China chemical safety case study: metals pollution in Dexing, Jiangxi Province. https://ipen.org/sites/default/files/documents/Case%20Study%20Report%20Beihai%202015r.pdf
Jarsjö J, Chalov SR, Pietroń J, Alekseenko AV, Thorslund J (2017) Patterns of soil contamination, erosion and river loading of metals in a gold mining region of northern Mongolia. Reg Environ Change 17(7):1991–2005. https://doi.org/10.1007/s10113-017-1169-6
Jiang YH, Zhao P, Zhou Q, Liao SY, Jin GD (2011) Petrogenesis and tectonic implications of Early Cretaceous S-and A-type granites in the northwest of the Gan-Hang rift, SE China. Lithos 121(1–4):55–73. https://doi.org/10.1016/j.lithos.2010.10.001
Jiangxi Provincial Bureau of Geology (1980) Hydrogeologic reconnaissance of the Jingdezhen region (In Chinese)
Karamalidis A, Dzombak D (2010) Surface complexation modeling: gibbsite. Wiley, New York
Khan MJ, Jones DL (2009) Effect of composts, lime and diammonium phosphate on the phytoavailability of heavy metals in a copper mine tailing soil. Pedosphere 19(5):631–641. https://doi.org/10.1016/S1002-0160(09)60158-2
Li G (2018) Practice of slope ecological restoration project in Fujiawu deposit of Dexing Copper Mine (in Chinese with English abstract). Copp Eng 6:14–16
Li T, Tao Q, Liang C, Shohag M, Yang X, Sparks DL (2013) Complexation with dissolved organic matter and mobility control of heavy metals in the rhizosphere of hyperaccumulator Sedum alfredii. Environ Pollut 182:248–255. https://doi.org/10.1016/j.envpol.2013.07.025
Liang Y, Xiao H, Liu X, Shi H (2019) The risk and phytotoxicity of metal(loid)s in the sediment, floodplain soil, and hygrophilous grasses along Le’an River. Int J Environ Sci Technol 17(4):1963–1974. https://doi.org/10.1007/s13762-019-02592-0
Lin W, Wu K, Lao Z, Hu W, Lin B, Li Y, Fan H, Hu J (2019) Assessment of trace metal contamination and ecological risk in the forest ecosystem of dexing mining area in northeast Jiangxi Province, China. Ecotoxicol Environ Saf 167:76–82. https://doi.org/10.1016/j.ecoenv.2018.10.001
Liu W, Wang Z, Wen X, Tang H (1999) The application of preliminary sediment quality criteria to metal contamination in the Le An River. Environ Pollut 105(3):355–366. https://doi.org/10.1016/S0269-7491(99)00041-X
Liu W, Coveney R, Chen J (2003) Environmental quality assessment on a river system polluted by mining activities. Appl Geochem 18(5):749–764. https://doi.org/10.1016/S0883-2927(02)00155-5
Liu G, Tao L, Liu X, Hou J, Wang A, Li R (2013) Heavy metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province, China. J Geochem Explor 132:156–163. https://doi.org/10.1016/j.gexplo.2013.06.017
Liu J, Wu J, Feng W, Li X (2020) Ecological risk assessment of heavy metals in water bodies around typical Copper Mines in China. Int J Environ Res Public Health 17(12):4315. https://doi.org/10.3390/ijerph17124315
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv 2(2):e1500323. https://doi.org/10.1126/sciadv.1500323
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ Health Perspect 116(3):278–283. https://doi.org/10.1289/ehp.10608
MH (Ministry of Health of the People’s Republic of China), SAC (Standardization Administration of the People’s Republic of China) 2006 Standards for drinking water quality (GB 5749-2006). Beijing: Standards Press of China
Mishra D, Kim DJ, Ralph DE, Ahn JG, Rhee YH (2008) Bioleaching of spent hydro-processing catalyst using acidophilic bacteria and its kinetics aspect. J Hazard Mater 152(3):1082–1091. https://doi.org/10.1016/j.jhazmat.2007.07.083
Nystrand MI, Österholm P (2013) Metal species in a Boreal river system affected by acid sulfate soils. Appl Geochem 31:133–141. https://doi.org/10.1016/j.apgeochem.2012.12.015
Nystrand MI, Österholm P, Yu C, Åström M (2016) Distribution and speciation of metals, phosphorus, sulfate and organic material in brackish estuary water affected by acid sulfate soils. Appl Geochem 66:264–274. https://doi.org/10.1016/j.apgeochem.2016.01.003
O’Neal SL, Zheng W (2015) Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep 2(3):315–328. https://doi.org/10.1007/s40572-015-0056-x
Pan H, Cheng Z, Zhou G, Yang R, Sun B, He L, Zeng D, Wang J (2017) Geochemical and mineralogical characterization of tailings of the Dexing copper mine, Jiangxi Province, China. Geochem Explor Environ Anal 17(4):334–344. https://doi.org/10.1144/geochem2016-457
Park I, Tabelin CB, Jeon S, Li X, Seno K, Ito M, Hiroyoshi N (2019) A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 219:588–606. https://doi.org/10.1016/j.chemosphere.2018.11.053
Pei H, Wang C, Wang Y, Yang H, Xie S (2019) Distribution of microbial lipids at an acid mine drainage site in China: insights into microbial adaptation to extremely low pH conditions. Org Geochem 134:77–91. https://doi.org/10.1016/j.orggeochem.2019.05.008
Peng B, Tang X, Yu C, Xie S, Xiao M, Song Z, Tu X (2009) Heavy metal geochemistry of the acid mine drainage discharged from the Hejiacun uranium mine in central Hunan, China. Environ Geol 57(2):421–434. https://doi.org/10.1007/s00254-008-1313-1
RoyChowdhury A, Sarkar D, Datta R (2015) Remediation of acid mine drainage-impacted water. Curr Pollut Rep 1(3):131–141. https://doi.org/10.1007/s40726-015-0011-3
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Bio/technology 13(5):468–474. https://doi.org/10.1038/nbt0595-468
Sjöstedt C, Andrén C, Fölster J, Gustafsson JP (2013) Modelling of pH and inorganic aluminium after termination of liming in 3000 Swedish lakes. Appl Geochem 35:221–229. https://doi.org/10.1016/j.apgeochem.2013.04.014
Tao L, Liu G, Liu X, Zhang C, Cheng D, Wang A, Li R (2014) Trace metal pollution in a Le’an River tributary affected by non-ferrous metal mining activities in Jiangxi Province, China. Chem Ecol 30(3):233–244. https://doi.org/10.1080/02757540.2013.861824
Teng Y, Ni S, Wang J, Zuo R, Yang J (2010) A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area, China. J Geochem Explor 104(3):118–127. https://doi.org/10.1016/j.gexplo.2010.01.006
Thorslund J, Jarsjo J, Chalov SR, Belozerova EV (2012) Gold mining impact on riverine heavy metal transport in a sparsely monitored region: the upper Lake Baikal Basin case. J Environ Monit 14(10):2780–2792. https://doi.org/10.1039/c2em30643c
Thorslund J, Jarsjö J, Wällstedt T, Mörth CM, Lychagin MY, Chalov SR (2014) Geochemical controls on the partitioning and hydrological transport of metals in a non-acidic river system. Hydrol Earth Syst Sci Discuss 11(8):9715–9758. https://doi.org/10.5194/hessd-11-9715-2014
Thorslund J, Jarsjö J, Wällstedt T, Mörth CM, Lychagin MY, Chalov SR (2016) Speciation and hydrological transport of metals in non-acidic river systems of the Lake Baikal basin: field data and model predictions. Reg Environ Change 17(7):2007–2021. https://doi.org/10.1007/s10113-016-0982-7
Tian XP, Li J (2000) Discussion on tailings—extracting and comprehensive utilization in Dexing Copper Mine. Geol Prospect 5
Tiberg C, Sjöstedt C, Persson I, Gustafsson JP (2013) Phosphate effects on copper (II) and lead (II) sorption to ferrihydrite. Geochim Cosmochim Acta 120:140–157. https://doi.org/10.1016/j.gca.2013.06.012
Tordoff G, Baker A, Willis A (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41(1–2):219–228. https://doi.org/10.1016/S0045-6535(99)00414-2
Wällstedt T, Björkvald L, Gustafsson JP (2010) Increasing concentrations of arsenic and vanadium in (southern) Swedish streams. Appl Geochem 25(8):1162–1175. https://doi.org/10.1016/j.apgeochem.2010.05.002
Wang L, Ji B, Hu Y, Liu R, Sun W (2017) A review on in situ phytoremediation of mine tailings. Chemosphere 184:594–600. https://doi.org/10.1016/j.chemosphere.2017.06.025
Wei F, Qi W, Sun Z, Huang Y, Shen Y (2002) Water and wastewater monitoring and analysis method (4th Edition, in Chinese). China Environmental Science Press, Beijing
Weng L, Temminghoff EJ, Lofts S, Tipping E, Van Riemsdijk WH (2002) Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol 36(22):4804–4810. https://doi.org/10.1021/es0200084
WHO (2006) Guidelines for drinking water quality, vol 1, 3rd edn. World Health Organization, Geneva
Wu A, Yin S, Wang H, Qin W, Qiu G (2009) Technological assessment of a mining-waste dump at the Dexing copper mine, China, for possible conversion to an in situ bioleaching operation. Bioresour Technol 100(6):1931–1936. https://doi.org/10.1016/j.biortech.2008.10.021
Wu Q (2018) Application of new technology for ecological restoration of abandoned metal mines. Copp Eng 6
Wu J, Teng Y, Lu S, Wang Y, Jiao X (2014) Evaluation of soil contamination indices in a mining area of Jiangxi, China. PLoS ONE 9(11):e112917. https://doi.org/10.1371/journal.pone.0112917
Wu R, Liao K, Deng YQ, Zhang JM, Lu JY (2020) Analysis of water quality characteristics of atmospheric precipitation in Jiangxi province and its influence on surface water (in Chinese with English abstract). Jiangxi Hydraul Sci Technol 46(5)
Xiao HY, Zhou WB, Zeng FP, Wu DS (2009) Water chemistry and heavy metal distribution in an AMD highly contaminated river. Environ Earth Sci 59(5):1023–1031. https://doi.org/10.1007/s12665-009-0094-5
Xiao HY, Zhou WB, Wu DS, Zeng FP (2011) Heavy metal contamination in sediments and floodplain topsoils of the Lean River catchment. China Soil Sediment Contam 20(7):810–823. https://doi.org/10.1080/15320383.2011.609200
Xue Q (2013) Geochemical characteristics and environmental assessment of the DeXing Cu Mine in Jiangxi province of China (in Chinese with English abstract). Doctoral thesis, Chengdu University of Technology
Xue Q, Zhao Y, Liu J, Zhang J, Guo K, Lu L (2012) Evolution of the environmental Quality and the early warning model research on heavy metals in DeXing copper mine (in Chinese with English abstract). Miner Depos 31(S1):495–496
Yang X (2011) The geological characteristics of Dexing Yinshan copper-lead-zinc deposit, mining exploration and evaluation work of deep resources. Central South University (in Chinese), pp 23–45
Yanguo T, Shijun N, Pengcheng J, Jian D, Chengjiang Z, Jinsheng W (2004) Eco-environmental geochemistry of heavy metal pollution in Dexing mining area. Chin J Geochem 23(4):349–358. https://doi.org/10.1007/BF02871307
Yi M (2019) Dynamic anlysis on environmental quallity of main river in the dexing copper mine from 2002 to 2017. Master thesis (in Chinese with English abstract), China University of Geosciences
Yu C, Lavergren U, Peltola P, Drake H, Bergbäck B, Åström ME (2014) Retention and transport of arsenic, uranium and nickel in a black shale setting revealed by a long-term humidity cell test and sequential chemical extractions. Chem Geol 363:134–144. https://doi.org/10.1016/j.chemgeo.2013.11.003
Yu Y, Wang H, Li Q, Wang B, Yan Z, Ding A (2016) Exposure risk of rural residents to copper in the Le’an River Basin, Jiangxi Province, China. Sci Total Environ 548–549:402–407. https://doi.org/10.1016/j.scitotenv.2015.11.107
Yu C, Berger T, Drake H, Song Z, Peltola P, Åström ME (2019) Geochemical controls on dispersion of U and Th in Quaternary deposits, stream water, and aquatic plants in an area with a granite pluton. Sci Total Environ 663:16–28. https://doi.org/10.1016/j.scitotenv.2019.01.293
Zhou Q, Jiang YH, Zhao P, Liao SY, Jin GD, Liu Z, Jia RY (2012) SHRIMP U-Pb dating on hydrothermal zircons: evidence for an Early Cretaceous epithermal event in the Middle Jurassic Dexing porphyry copper deposit. Southeast China Econ Geol 107(7):1507–1514. https://doi.org/10.2113/econgeo.107.7.1507
Zhou Z, Chen Z, Pan H, Sun B, Zeng D, He L, Yang R, Zhou G (2018) Cadmium contamination in soils and crops in four mining areas, China. J Geochem Explor 192:72–84. https://doi.org/10.1016/j.gexplo.2018.06.003
Acknowledgements
We thank the Scientific Research Fund of Jiangxi Provincial Education Department (contract BJJ180384 to S.X.) and the Swedish Research Council Formas (contract 2020-01004 to C.Y.) for financial support.
Funding
Open access funding provided by Linnaeus University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Jing Chen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, S., Yu, C., Peng, B. et al. A re-assessment of metal pollution in the Dexing mining area in Jiangxi province, China: current status, hydro-geochemical controls, and effectiveness of remediation practices. Int. J. Environ. Sci. Technol. 19, 10707–10722 (2022). https://doi.org/10.1007/s13762-021-03887-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03887-x