Abstract
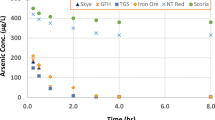
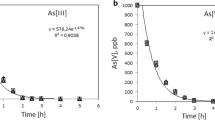
Recently it has been unearthed that many potable water sources around the world are contaminated with arsenic, which has got long-term health hazards for the consumers. Among different arsenic removal techniques, adsorption is widely used and easily achievable. Through the use of suitable adsorbent, arsenic can be significantly removed from potable water, which is a dire need for many distant communities where there is scarcity of alternative potable water source. This paper presents arsenic removal characteristics of different synthetic and natural sand samples through adsorption. To replicate the Skye sand which was found to be effective in removing arsenic, two synthetic sand samples (one coated with iron and the other coated with aluminium) were prepared in the laboratory. Different combinations of these samples were tested through batch experiments to evaluate their arsenic removal efficiencies under different conditions. It is found that among the synthetic adsorbents, the ones dried at 80 °C (during their preparation) showed the highest arsenic removal efficiencies. Also, among all the samples, iron oxide-coated sand (IOCS) showed the highest arsenic removal efficiencies, varying from 71 to 100% depending on the amount of doses, whereas with the use of natural Skye sand with the same variation in doses, arsenic removal efficiencies varied from 26 to 90%.




Similar content being viewed by others
References
Altundogan H, Altundogan S, Tumen F, Bildik M (2002) Arsenic adsorption from aqueous solutions by activated red mud. Waste Manage 22:357–363
Badruzzaman M, Westerhoff P, Knappe DR (2004) Intraparticle diffusion and adsorption of arsenate onto granular ferric hydroxide (GFH). Water Res 38(18):4002–4012
Bhardwaj A, Rajput R, Misra K (2019) Status of arsenic remediation in India. In: Ahuja S (ed) Advances in water purification techniques, Chapter 9. Elsevier, Amsterdam, ISBN: 9780128147900, https://doi.org/10.1016/B978-0-12-814790-0.00009-0.
Bertocchi A, Ghiani M, Peretti R, Zucca A (2006) Red Mud and fly ash for the remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn. J Hazard Mater B 134:112–119
Bundschuh J, Bhattacharya P, Sracek O, Mellano MF, Ramírez AE, Storniolo AdR, Martín RA, Cortés J, Litter MI, Jean J-S (2011) Arsenic removal from groundwater of the Chaco-Pampean Plain (Argentina) using natural geological materials as adsorbents. J Environ Sci Health A 46(11):1297–1310
Deliyanni E, Bakoyannakis D, Zouboulis A, Matis K (2003) Sorption of As(V) ions by akaganéite-type nanocrystals. Chemosphere 50:155–163
DeMarco M, SenGupta A, Greenleaf J (2003) Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res 37:164–176
Deschamps E, Cimnelli V, Holl W (2005) Removal of As(III) and As(V) from water using a natural Fe and Mn enriched sample. Water Res 39:5212–5220
Dutta P, Ray A, Sharma V, Millero F (2004) Adsorption of arsenate and arsenite on titanium dioxide suspensions. J Colloid Interface Sci 278:270–275
Genc-Fuhrman H, Bergnhoj H, McConchie D (2005) Arsenate removal from water using sand-red mud columns. Water Res 39:2944–2954
Ghimire K, Inoue K, Yamaguchi H, Makino K, Miyajima T (2003) Adsorptive separation of arsenate and arsenite anions from aqueous medium by using orange waste. Water Res 34:4945–4953
Hlavay J, Polyak K (2005) Determination of surface properties of ironhydrocide-coated alumina adsorbent prepared for removal of arsenic from drinking water. J Colloid Interface Sci 284:71–77
Hu X, Ding Z, Zimmerman AR, Wang S, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Imteaz MA, Ahmed MY, Khan MS, Ahsan A (2016) A simple clogging and back-washing efficiency model for filtration of arsenic-contaminated water. Desalin Water Treat 57(26):12237–12243
Imteaz MA, Ahsan A, Kaur P (2020) Improved clogging efficiency model for filtration of arsenic-contaminated water. Proc ICE Water Manage. https://doi.org/10.1680/jwama.19.00077
Imteaz MA, Arulrajah A (2019) Removal of heavy metals from contaminated foundry sand through repeated soil-washing. Int J Sustain Eng. https://doi.org/10.1080/19397038.2019.1657982
Imteaz MA, Khan SA, Ahsan A (2019) Modifications of a simple clogging and back-washing efficiency model for Arsenic filters. Proc ICE Water Manage 172(6):284–290. https://doi.org/10.1680/jwama.17.00077
Jekel M (1994) Removal of arsenic in drinking water treatment. In: Nriagu JO (ed) Arsenic in the environment. Part I: cycling and characterization. Wiley, New York.
Jing C, Meng X, Liu S, Baidas S, Patraju R, Christodoulatus C, Korfiatis C (2005) Surface komlexation of organic arsenic on nanocrystalline titanium dioxide. J Colloid Interface Sci 290:14–21
Katsoyiannis IA, Zouboulis AI (2004) Application of biological processes for the removal of arsenic from groundwaters. Water Res 38(1):17–26
Khan SA (2004) Field-testing of improved IHE Family Filter in Bangladesh, MSc Thesis, UNESCO-IHE Institute for Water Education, Delft, Netherlands.
Khan SA (2017) Removal of arsenic from drinking water by natural adsorbents. PhD Thesis, Monash University, Melbourne, Australia.
Kuan W-H, Lo S-L, Wang MK, Lin C-F (1998) Removal of Se(IV) and Se(VI) from water by aluminum-oxide-coated sand. Water Res 32(3):915–923
Kundu S, Gupta A (2005) Analysis and modeling of fixed bed column operations on As(V) removal by adsorption onto iron oxide-coated cement (I0CC). J Colloid Interface Sci 290:52–60
Lakshmipathiraj P, Narasimhan BRV, Prabhakar S, Bhaskar Raju G (2006) Adsorption of arsenate on synthetic goethite from aqueous solutions. J Hazard Mater 136(2):281–287
Lenoble V, Laclautre C, Serpaud B, Deluchat V, Bollinger J (2004) As(V) retention and As(III) simultaneous oxidation and removal on Mn02 loaded polystyrene resin. Sci Total Environ 326:197–207
Loukidou M, Matis K, Zouboulls A, Liakopoulou-Kyriakidou M (2003) Removal of As(V) from wastewaters by chemically modified fungal biomass. Water Res 37:4544–4552
Maiti A, Thakur BK, Basu JK, De S (2013) Comparison of treated laterite as arsenic adsorbent from different locations and performance of best filter under field conditions. J Hazard Mater 262:1176–1186
Manju G, Raji C, Anirudhan T (1998) Evaluation of coconut husk carbon for the removal of As from water. Water Res 32:3062–3070
Matis K, Lehmann M, Zouboulis A (1999) Modelling sorption of metals from aqueous solution onto mineral particles: the case of arsenic ions and goethite ore. In: Misaelides et al. (eds) Natural microporous materials in environmental technology. Kluwer, The Netherlands, pp. 463–472.
Nicomel NR, Leus K, Folens K, Voort PVD, Laing GD (2016) Technologies for Arsenic removal from water: current status and future perspectives. Int J Environ Res Public Health 13(62):1–24
Neumann A, Kaegi R, Voegelin A, Hussam A, Munir AKM, Hug SJ (2013) Arsenic removal with composite iron matrix filters in Bangladesh: a field and laboratory study. Environ Sci Technol 47:4544–4554
Omeroglu P (2001) Use of iron coated sand for Arsenic removal. MSc thesis (SEE 131), UNESCO-IHE, Delft, The Netherlands.
Patanayak J, Mondal K, Mathew S, Lalvani S (2000) A parametric evaluation of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon 38:589–596
Pokhrel D, Viraraghavan T (2006) Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res 40(3):549–552
Rau I, Gonzalo A, Valiente M (2003) Arsenic (V) adsorption by imobilized iron mediation. Modeling of the adsorption process and influence of interfering anions. React Funct Polym 54:85–94
Roy P, Mondal NK, Bhattacharya S, Das B, Das K (2013) Removal of arsenic(III) and arsenic(V) on chemically modified low-cost adsorbent: batch and column operations. Appl Water Sci 3(1):293–300
Shakoor MB, Nawaz R, Hussain F, Raza M, Ali S (2017) Human health implications, risk assessment and remediation of As-contaminated water: a critical review. Sci Total Environ 601(602):756–769
Singh T, Pant K (2004) Equilibrium, kinetics and thermodynamic studies for adsorption of As (III) on activated alumina. Sep Purif Technol 36:139–147
Sperlich A, Werner A, Genz A, Amy C, Worch E, Jekel I (2005) Breakthrongh behavior of granular ferric hydroxide (C PH) fixed- bed adsorption filters: modeling and experimental approaches. Water Res 39:1190–1198
Thirunavukkarasu OS, Viraraghavan T, Subramanian KS (2003) Arsenic removal from drinking water using iron oxide-coated sand. Water Air Soil Pollut 142(1–4):95–111
Vaclavikova M, Matik M, Jakabsky S, Hredzak S (2005) Preparation and sorption properties of Fe-nanomaterials for removal of arsenic from waters. In: Book of abstract of NATO CCMS on clean products and processes, Norway, p 13.
Xu Z, Li Q, Gao S, Shang JK (2010) As(III) removal by hydrous titanium dioxide prepared from one-step hydrolysis of aqueous TiCl4 solution. Water Res 44(19):5713–5721
Zeng L (2003) A method for preparing silica-containing iron(III) oxide adsorbents for arsenic removal. Water Res 37:4351–4358
Zhang W, Singh P, Paling E, Delides S (2004) Arsenic removal from contaminated water by natural iron ores. Miner Eng 17(4):517–524
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: M. Shabani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, S.A., Imteaz, M.A. Batch experiments on arsenic removal efficiencies through adsorption using synthetic and natural sand samples. Int. J. Environ. Sci. Technol. 18, 2357–2364 (2021). https://doi.org/10.1007/s13762-020-02999-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02999-0