Abstract
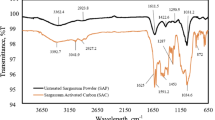
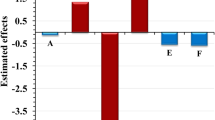
In this examination, the proficiency of alga biomass Sargassum muticum for simultaneous uptake of methylene blue (MB) and lead (II) (Pb2+) ions from aqueous solution is investigated. The brown alga is surveyed by scanning electron microscopy, Fourier transform infrared spectroscopy (FTIR) and nitrogen adsorption–desorption procedures. Impact of operating variables (brown alga mass, contact time and initial MB and Pb+2-ion concentration) on the simultaneous contaminants uptake is performed by means of central composite design and upgraded through response surface method (RSM). Accuracy of the equation procured by RSM is checked by the variance investigation and computing of determination coefficient (R2) among predicted and the experimental values of simultaneous noxious substances removal. Bon accord among empirical and envisaged values is commented. The optimum conditions are settled at contact time (30 min), 39 mg L−1 for MB and 30 mg L−1 for Pb2+ ions and brown alga mass (0.3 g) to reach maximum retention percentage. Adsorption kinetic depicted three phases intra-particle diffusion mode and coordinated the pseudo-second-order design very well. Batch equilibrium results reveal that MB and Pb2+ ions biosorption onto alga biomass could be all around portrayed by Langmuir isotherm model contrasted with Freundlich approach. In any case, removal percentage elaborated from column studies is inferior to those computed from batch technique. Column can be recouped and reutilized even after 5-adsorption–desorption cycles. X-ray photoelectron spectroscopy and FTIR scrutinies uncovered that hydroxyl, carbonyl and amino functional groups are responsible for the biosorption of both pollutants onto non-living brown alga. Previous tests achieved on paint wastewater demonstrated that the invasive biomass Sargassum muticum could be an effective and promising biosorbent for dye/metal wastewater treatment.
Graphic abstract







Similar content being viewed by others
References
Adamczuk A, Kołodyńska D (2015) Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem Eng J 274:200–212
Ahmaruzzaman M (2011) Industrial wastes as low-cost potential adsorbents for the treatmentof wastewater laden with heavy metals. Adv Colloid Interface Sci 166:36–59
Arumugam N, Chelliapan S, Kamyab H, Thirugnana S, Othman N, Nasri N (2018) Treatment of wastewater using seaweed: a review. Int J Environ Res Public Health 15:2851–2868
Carro L, Barriada JL, Herrero R, Sastre de Vicente ME (2013) Surface modifications of Sargassum muticum algal biomass for mercury removal: a physicochemical study in batch and continuous flow conditions. Chem Eng J 229:378–387
Carro L, Barriada JL, Herrero R, Sastre de Vicente ME (2011) Adsorptive behaviour of mercury on algal biomass: competition with divalent cations and organic compounds. J Hazardous Mater 192:284–291
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons. Adsorption of organic vapors. Zh Fiz Khim 21:1351–1362
Esmaeli A, Jokar M, Kousha M, Daneshvar E, Zilouei H, Karimi K (2013) Acidic dye wastewater treatment onto a marine macroalga, Nizamuddina zanardini (Phylum: Ochrophyta). Chem Eng J 217:329–336
Freundlich H (1928) Colloid and capillary chemistry. Oxford, Elsevier
Ghaedi M, Mazaheri H, Khodadoust S, Hajati S, Purkait MK (2015) Application of central composite design for simultaneous removal of methylene blue and Pb2+ ions by walnut wood activated carbon. Spectrochim Acta A Mol Biomol Spectrosc 135:479–490
Guler UA, Sarioglu M (2013) Single and binary biosorption of Cu(II), Ni(II) and methylene blue by raw and pretreated Spirogyra sp: equilibrium and kinetic odelling. J Environ Chem Eng 1:369–377
Hannachi Y, Rezgui A, Dekhil AB, Boubaker T (2015) Removal of Cadmium (II) from aqueous solutions by biosorption onto the brown macroalga (Dictyota dichotoma). Desalination Water Treat 54:1663–1673
Lagergren S (1898) Zur theorie der sogenannten, adsorption geloster stoffe. Kungliga Sevenska Ventenskapasa kademiens, Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica, and platinum. J Am Chem Soc 40:1361–1403
Mallampati R, Xuanjun L, Adin A, Valiyaveettil S (2015) Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water. ACS Sustain Chem Eng 3:1117–1124
Moyo M, Chikazaza L (2013) Bioremediation of lead(II) from polluted wastewaters employing sulphuric acid treated maize tassel biomass. Am J Anal Chem 4:689–695
Nas MS, Calimli MH, Burhan H, Yılmaz M, Mustafov SD, Sen F (2019a) Synthesis, characterization, kinetics and adsorption properties of Pt-Co@GO nano-adsorbent for methylene blue removal in the aquatic mediums using ultrasonic process systems. J Mol Liq 296:112100
Nas MS, Kuyuldar E, Demirkan B, Calimli MH, Demirbaş O, Sen F (2019b) Magnetic nanocomposites decorated on multiwalled carbon nanotube for removal of Maxilon Blue 5G using the sono-Fenton method. Sci Rep 9:10850
Ozudogru Y, Merdivan M, Goksan T (2017) Removal of methylene blue from aqueous solutions by brown alga Cystoseira barbata. Desalination Water Treat 62:267–272
Petrovič A, Simonič M (2016) Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int J Environ Sci Technol 13:1761–1780
Raize O, Argaman Y, Yannai S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87:451–458
Rajapaksha P, Chandra S, Power A, Chapman J (2018) Graphene, electrospun and granular activated carbon membranes for eliminating heavy metals, pesticides and bacteria in water and wastewater treatment processes. Analyst 143:5629–5645
Ratna Kumari A, Sobha K (2016) Removal of lead by adsorption with the renewable biopolymer composite of feather (Dromaius novaehollandiae) and chitosan (Agaricus bisporus). Environ Technol Inno 6:11–26
Rice EW, Baird RB, Eaton AD (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, American Water Works Association, Water Environment Federation
Rubin E, Rodríguez P, Herrero R, Sastre de Vicente ME (2006) Biosorption of phenolic compounds by the brown alga Sargassum muticum. J Chem Technol Biotechnol 81:1093–1099
Rubin E, Rodriguez P, Herrero R, Cremades J, Barbara I, Sastre de Vicente ME (2005) Removal of Methylene Blue from aqueous solutions using as biosorbent Sargassum muticum: an invasive macroalga in Europe. J Chem Technol Biotechnol 80:291–298
Ruiqi F, Zimo L, Zhuoxing W, Shams AB, Lou Z, Wang Z, Xinhua X (2016) Adsorptive removal of Pb(II) by magnetic activated carbon incorporated with amino groups from aqueous solutions. J Taiwan Inst Chem E 62:247–258
Taamneh Y, Sharadqah S (2016) The removal of heavy metals from aqueous solution using natural Jordanian zeolite. Appl Water Sci 7:2021–2028
Vilardi G, Palma LD, Verdone N (2018) Heavy metals adsorption by banana peels micro-powder: equilibrium odelling by non-linear models. Chin J Chem Eng 26:455–464
Weber WJ, Morris JC (1963) Kinetics of adsorption carbon from solutions. J sanit Eng Div 89:31–60
Xiong R, Wang Y, Zhang X, Lu C (2014) Facile synthesis of magnetic nanocomposites of cellulose@ultrasmall iron oxide nanoparticles for water treatment. RSC Adv 4:22632–22641
Yang C, Girma A, Lei T, Liu Y, Ma C (2016) Study on simultaneous adsorption of Zn(II) and methylene blue on waste-derived activated carbon for efficient applications in wastewater treatment. Cogent Environ Sci 2:1–9
Zhao Y, Tian G, Duan X, Liang X, Meng J, Liang J (2019) Environmental applications of diatomite minerals in removing heavy metals from water. Ind Eng Chem Res 58:11638–11652
Zazouli MA, Azari A, Dehghan S, Salmani Malekkolae R (2016) Adsorption of methylene blue from aqueous solution onto activated carbons developed from eucalyptus bark and Crataegus oxyacantha core. Water Sci Technol 74:2021–2035
Acknowledgments
The authors are thankful to the Belgorod State Technological University (Russia) for financial support to this study and greatly acknowledge Prof. N.A. Shappovalov for stimulating discussion and performing XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare a no conflict of interest.
Additional information
Communicated by Fatih ŞEN.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hannachi, Y., Hafidh, A. Biosorption potential of Sargassum muticum algal biomass for methylene blue and lead removal from aqueous medium. Int. J. Environ. Sci. Technol. 17, 3875–3890 (2020). https://doi.org/10.1007/s13762-020-02742-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02742-9