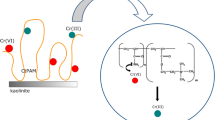
Abstract
The main purpose of the study was to examine lead(II) ion immobilization on the kaolinite surface and determine the effect of soil flocculant (macromolecular compound) on this process. For this reason, the adsorption measurements of anionic polyacrylamide and/or Pb(II) ions on the kaolinite surface were performed. Moreover, the impact of heavy metal on electrokinetic and stability properties of kaolinite/polyacrylamide/supporting electrolyte system was determined. It was done based on the results of potentiometric titration, electrophoretic mobility, and absorbance measurements. The most important conclusions of the study were as follows: (1) kaolinite is an effective Pb(II) adsorbent when ion concentration in the environment equals 1 or 10 ppm (it adsorbs 80% and 72% of the ions, respectively), (2) when Pb(II) concentration in the environment is 100 ppm, kaolinite adsorbs only 29% of the ions, and (3) anionic polyacrylamide may strengthen the Pb(II) adsorption on the kaolinite surface (in the presence of 100 ppm AN PAM 30% and 1 ppm Pb(II), even 96% of the ions are adsorbed). Due to the above process, the bioavailability of heavy metal for organisms is considerably limited.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environment pollution with heavy metals is mainly associated with continuous development of industry and technology. Many heavy metals are present in soils, especially those located near industrial plants and roads. Some of them are washed out of the ground and, as a consequence, pollute groundwater significantly (Niesiobędzka 2003). Heavy metals have a negative impact on the organism growth, and due to this fact, it is very important to develop methods that would reduce the amount or bioavailability of heavy metals in the environment (Ociepa-Kubicka and Ociepa 2012).
One of the ways limiting the heavy metals negative impact is their immobilization by various substances, both organic and inorganic, which are added to the soil separately or simultaneously. These compounds bind toxic forms on their surfaces, which leads to reduction in their bioavailability for plants and animals. Heavy metal immobilization is based on various processes, including adsorption, metal precipitation on the solid surface, ion exchange, and formation of stable heavy metal–organic ligand complexes (Lwin et al. 2018; Gadepalle et al. 2007). Thanks to this phenomenon, heavy metals do not enter the food chain. Moreover, smaller quantities of toxic substances end up in groundwater (Alkorta et al. 2004).
Studies on soil additives that can immobilize heavy metals are being carried out by many scientists. Mahmoud and El-Kader (2013, 2015) used phosphogypsum and compost to immobilize lead, cadmium and zinc, whereas Oste et al. (2002) bind the same heavy metals using synthetic and natural zeolites. Houben et al. (2012) investigated CaCO3, iron grit, fly ash, and bone meal as substances limiting Cd, Zn, and Pb leaching. Tomczyk et al. (2019) showed that the biochar obtained from wood wastes effectively binds copper on its surface. Wang et al. (2017) performed contaminated soil remediation using various biochars (from pig manure and corn straw) and bacterial strain B. subtilis B38. In turn, Fahmi et al. (2018) examined Cd and Pb leaching from contaminated soil amended with different size of biochar. The use of above-mentioned substances as soil additives is usually low cost (Houben et al. 2012).
Clay minerals are also used in remediation of heavy metal-polluted soils. These inorganic materials may increase soil pH value as well as decrease chemical extractable fractions and bioavailability of heavy metals in soils. Bentonite, sepiolite, and palygorskite are usually used to improve the soil environment quality (Xu et al. 2017). In this paper, another clay mineral—kaolinite—was the subject to research. The sorption properties of this material relative to Pb(II) ions were examined. Moreover, the influence of commonly used soil flocculant—anionic polyacrylamide (AN PAM), on the lead sorption on the kaolinite surface, was investigated. Potentiometric titration method and electrophoretic mobility measurements allowed determination of the electrokinetic properties of the kaolinite particles in the absence and presence of Pb(II)/AN PAM. As a result, the structure of electrical double layer (EDL) of clay mineral particles with and without selected additives was proposed. The research included also stability measurements of the kaolinite suspension without and with Pb(II) and/or AN PAM. In these experiments, the changes in system turbidity over time were measured. In this way, solid aggregation after macromolecular compound and/or heavy metal addition was established. It was checked whether soil flocculant retains its structure-forming properties in the lead(II) presence.
The presented results are of high scientific value. Based on their analysis, the authors indicated: (1) whether kaolinite can be used as a soil additive increasing the lead immobilization; (2) whether polymeric substance enhances the Pb(II) binding process; (3) whether the soil flocculant retains its structural-forming and anti-erosive ability in the heavy metal presence. The data presented refers directly to practical use, e.g., in agriculture, during soil remediation. Lead gets into the environment through transport and industrial emissions. At high concentrations, it causes photosynthesis, cell division, and nitrogen metabolism disorders as well as has strong embryotoxic, carcinogenic and mutagenic impact on organisms (Szymański 2009). Therefore, effective Pb(II) immobilization is necessary for the proper functioning of plants and animals.
The experiments were performed in the second half of 2018, at Maria Curie-Skłodowska University in Lublin. The result interpretation was also made in Institute of Agrophysics PAS in Lublin.
Materials and methods
Materials
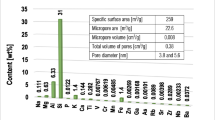
Kaolinite—1:1 clay mineral (Sigma-Aldrich, CAS 1318-74-7)—was used in the study as an adsorbent. Specific surface area and porosity of the solid were determined applying a nitrogen adsorption/desorption method (Micromeritics ASAP 2020 analyzer). Table 1 contains the obtained parameters.
An anionic polyacrylamide (AN PAM) with different ionic group content, i.e., 5% and 30%, was delivered by Korona. AN PAM containing 5% ionizable groups are marked AN PAM-1, whereas that containing 30%—AN PAM-2. The weight average molecular weight of the polymer samples was equal to 13 000 and 14 000 kDa, respectively. Using a potentiometric titration, the anionic PAM pKa values and degrees of carboxylic group ionization (α) were determined. The pKa parameter of AN PAM-1 is equal to 3.3, and the dissociation degree is as follows: at pH 3—33.4%, pH 5—98%, pH 7—99.98%, and pH 9—99.99%. In the case of AN PAM-2, the pKa value equals 3.2 and the dissociation degree is: at pH 3—38.7%, pH 5—98.7%, pH 7—99.98%, and pH 9—99.99%. The above results indicated that at pH higher than or equal to 5 almost all carboxylic groups in PAM macromolecules are dissociated.
Methods
In all experiments, Pb(II) ions were added to the systems as lead chloride (PbCl2).
Adsorption experiments were performed at 25 °C, pH 5, using 0.001 mol/L sodium chloride (NaCl) as a supporting electrolyte. The amount of AN PAM or lead(II) ions on the kaolinite surface was calculated from the difference between the polymer or heavy metal concentration in the solution before and after its adsorption. At the beginning, several probes were prepared by adding 0.1 g of kaolinite to appropriate solution containing Pb(II) and/or AN PAM and supporting electrolyte (solid/liquid ratio in the probes was 0.1:10). Lead(II) adsorption isotherms were prepared using Pb(II) concentrations in the range from 1 to 200 ppm, whereas AN PAM adsorbed amounts on the kaolinite surface were measured for polymer initial concentration equal to 100 ppm. AN PAM impact on Pb(II) immobilization by kaolinite was examined in the systems consisting of 100 ppm of AN PAM and 1, 10, or 100 ppm of Pb(II) ions. The above Pb(II) concentrations were chosen to reflect environmental pollution with this heavy metal [permissible lead(II) concentration in the environmental probes equals 0.1 ppm in wastewater and 0.01 ppm in drinking water (according to the Polish Regulation)]. AN PAM concentration of 100 ppm is the optimal one, at which the polymer exhibits strong flocculating properties. The appropriate pH value of the studied suspensions were adjusted with a pH meter (Beckman Instruments) using 0.1 mol/L NaOH and 0.1 mol/L HCl. Adsorption was carried out under conditions of continuous shaking (water bath OLS 200, Grant) with speed 250 rpm, for 24 h. After the process completion, the samples were centrifuged (20 min, 10,000 rpm) using a microcentrifuge (MPW Med. Instruments), and the obtained supernatants were collected for further analysis. AN PAM concentration was determined based on the spectrophotometric method (UV–Vis spectrophotometer Carry 1000; Varian) with hyamine 1622 (N-benzyl-N,N-dimethyl-2-{2-[4-(2,4,4-trimethylpentan-2-yl)ethoxy}ethanaminium chloride), proposed by Crummett and Hummel (1963). This method is based on AN PAM–hyamine complex formation, which generates white color of the examined solutions. Absorbance was measured 15 min after hyamine addition, at the wavelength 500 nm. Pb(II) concentration was measured using the lead(II) complexation with 4-(2-pyridylazo)-rezorcinol (PAR) in an ammonium buffer (Dagnall et al. 1965). In this method, red-colored chelate complexes (Pb:PAR ratio is 1:1) are formed and the absorbance of studied systems is measured at the wavelength 520 nm. AN PAM and Pb(II) adsorbed amounts were established using calibration curves representing the dependence of absorbance on the polymer or heavy metal concentration. A single result of AN PAM/Pb(II) adsorbed amount was the average of three replicates. The error did not exceed 5%.
Pb(II) adsorption isotherm data were modeled using the Langmuir (Eq. 1) and Freundlich (Eq. 2) equations (Ościk 1969):
where KF (mg/g)—the Freundlich isotherm constant (the measure of adsorption capacity of the adsorbent), and KL—Langmuir isotherm constant, qe (mg/g)—the equilibrium adsorption capacity, Ce—the equilibrium liquid phase concentration, Qm—the maximum adsorption capacity in Langmuir model, 1/n—the Freundlich parameter (the measure of adsorption intensity).
Kinetics measurement of lead(II) adsorption were carried out as follows. Several probes (10 ml) were prepared by adding 0.1 g of kaolinite to the solution containing 10 ppm of lead(II) ions (or 100 ppm of AN PAM) and 0.001 mol/L of supporting electrolyte. After pH adjustment (pH 5), the adsorption process was started. It was conducted under continuous shaking conditions (water bath OLS 200, Grant) for 5, 10, 20, 30, 40, 50, 60, 90, 120, 180, and 240 min. The lead(II) concentration was determined using the heavy metal reaction with PAR (Dagnall et al. 1965), in turn AN PAM concentration—based on the polymer reaction with hyamine 1622 (Crummett and Hummel 1963). The results were modeled using pseudo-first-order (Eq. 3) and pseudo-second-order equations (Ho and McKay 1998, 2002):
where qe—the adsorbed amount at equilibrium, qt—the adsorbed amount after time ‘t’, k1 (1/min) and k2 (g/mg min)—the equilibrium rate constants.
The solid surface charge (σ0) as a function of solution pH value as well as point of zero charge (pHpzc) of kaolinite, in the absence and presence of additives, were determined by potentiometric titration method. For the systems containing Pb(II) ions, the measurements were performed in the pH range 3–5 (because lead hydroxide precipitation occurs at higher pH values), whereas for the systems containing only polymer and supporting electrolyte, they were performed in the pH range 3–9. Potentiometric titration was carried out for the kaolinite suspensions prepared in supporting electrolyte (0.001 mol/L NaCl) containing: (1) no additives, (2) AN PAM 5% 100 ppm, (3) AN PAM 30% 100 ppm, (3) Pb(II) ions 1 ppm, (4) AN PAM 5% 100 ppm and Pb(II) ions 1 ppm, and (5) AN 30% 100 ppm and Pb(II) ions 1 ppm. The suspensions (containing 0.1 g of mineral) were titrated with 0.1 mol/L NaOH in the measuring set consisting of: thermostated Teflon vessel (thermostat RE 204, Lauda), glass and calomel electrodes (Beckman Instruments), pH meter PHM 240 (Radiometer), automatic microburette (Dosimat 765, Methrom), and computer. The solid surface charge density was calculated using the special program Titr_v3 developed by Janusz (1994) using the following equation:
where ΔV—the difference in the base volume added to a suspension and a supporting electrolyte solution that leads to the specific pH value, c—the base concentration, F—the Faraday constant, m—the solid mass in the suspension, S—the solid surface area.
Kaolinite zeta potential (ζ) was determined based on electrophoretic mobility of particles measured using Zetasizer Nano ZS (Malvern Instruments) and universal dip cell. The software calculated the ζ values using Henry equation (Hunter 1981):
where Ue—the electrophoretic mobility, ε—the dielectric constant, ε0—the electric permeability of vacuum, ζ—the zeta potential, η—the viscosity, f(κa)—the Henry function.
The kaolinite suspensions in 0.001 mol/L NaCl, without and with AN PAM or lead(II) ions, were prepared by adding 0.1 g of mineral to 100 ml of the appropriate solution. AN PAM concentration in the probes equals 100 ppm, whereas the Pb(II) one—1 ppm. Each suspension was sonicated for 3 min (ultrasonicator XL 2020, Misonix) and separated into several parts, in which different pH value was adjusted. Zeta potential of solid particles in the Pb(II) ion presence was determined in the pH range 3–5 to avoid the lead hydroxide formation, whereas in the presence of only polymer and supporting electrolyte—in the pH range 3–9.
The clay mineral suspension stability without and with AN PAM/Pb(II) was monitored using a spectrophotometer UV/Vis (spectrophotometer Carry 1000, Varian). This method corresponds with turbidity measurement techniques—the registered absorbance reflects the turbidity of examined system. If the turbidity does not change over time, the recorded absorbance also remains at a constant level. This demonstrates the high stability of the system. On the other hand, if the system turbidity changes significantly over time, then the decrease in absorbance is also visible. Solid particles fall to the bottom of the vessel, the suspension gradually clarifies, which indicates low stability of the system (Szewczuk-Karpisz et al. 2019; Wisniewska et al. 2018a, b; Grządka and Matusiak 2017; Birdi 2003). The absorbance was measured at wavelength (λ) equal to 500 nm, which was selected based on the spectrum determination for kaolinite suspension (of white color). A single measurement lasted 3 h, and the absorbance was recorded 10, 20, 30, 60, 90, 120, 150, and 180 min after measurement start. To prepare examined probes, 0.1 g of kaolinite was added to the 10 ml of supporting electrolyte solution containing or non-containing polymer/metal ions, and the pH value (pH 5) was adjusted.
Results and discussion
Specific surface area and porosity of kaolinite
Specific surface area and porosity of kaolinite were established based on the method of low-temperature nitrogen adsorption/desorption isotherms. In this technique, a simple multilayer adsorption model from the gas phase on the adsorbent surface is used (BET isotherm) for specific surface area (SBET) determination. Pore-size distribution is calculated using BJH method. Total pore volume (Vp) is obtained based on the adsorbed amount of liquid nitrogen at a relative pressure p/p0 − 0.99. In turn, mean pore diameter (D) is calculated from the equation D = 4Vp/SBET. Figure 1 presents the obtained N2 isotherms and BJH pore-size distribution (PSD). The examined material is characterized by relatively low porosity parameters, i.e., its specific surface area equals 8.02 m2/g, whereas total pore volume—0.0287 cm3/g. The observed isotherms are of IV type with H3 hysteresis (Fig. 1a), which indicates multilayer adsorption on mesopores (Anovitz and Cole 2015; Numbonui Ghogomu et al. 2014; Ndi et al. 2015). The H3 hysteresis is typical for slit-like pores and/or panel-shaped particles (Wang et al. 2012). The mesoporous character of kaolinite is confirmed in the diagram of BJH pore-size distribution (Fig. 1b). There are two groups of mesopores: the first of average diameter equal to 3.8 nm and the second one—11.7 nm.
Pb(II) ion and AN PAM adsorption mechanism on the kaolinite surface
Figure 2a presents the Pb(II) ions adsorption kinetics on the kaolinite surface, whereas in Table 2, calculated kinetics parameters are summarized. These results show that after about 1 h, the equilibrium state is reached. The obtained data were fitted to the pseudo-second-order model with R2 value equal to 0.9883. The calculated qe values (qe,cal) and experimental (qe,exp) were very similar (qe,cal = 0.77 mg/g; qe,exp = 0.72 mg/g). This means that Pb(II) ion adsorption on kaolinite involves chemisorption (Wang et al. 2007; Oliveira et al. 2008) based on electron sharing or exchange between Pb(II) ions and the clay mineral. Low R2 parameter for kinetics data fitting to the pseudo-first-order model may indicate that during adsorption one heavy metal ion interacts with one active site. Figure 2b presents adsorption isotherms of Pb(II) ions on the kaolinite surface. These data were fitted to the Langmuir model with R2 value of 0.9993 (Table 3). This indicates that the Pb(II) ion monolayer is formed on the clay mineral surface, which is characterized by uniform adsorption energy. A single ion interacts with one active site and there are no interactions between adsorbed heavy metal ions (Ościk 1969; Paderewski 1999; Kielcew 1980; Zhang et al. 2009). Due to electrostatic interactions between heavy metal ions and solid particles, lead(II) ions have a strong affinity for the kaolinite surface. Potentiometric titration showed that the pHpzc (point of zero charge) value of the examined mineral equals 3.37, which means that at pH > 3.37 the concentration of negative surface groups (–SO−) is higher than that of positive ones (–SOH2+). Thus, at pH 5, there is a clear electrostatic attraction between positive Pb(II) ions and negative kaolinite surface. The measured Pb(II) adsorbed amounts were as follows: 0.08 mg/g (for the lead initial concentration equal to 1 ppm), 0.72 mg/g (initial concentration 10 ppm), and 2.89 mg/g (initial concentration 100 ppm). This means that the kaolinite adsorbs lead(II) ions with efficiency: 80% (for the lead initial concentration equal to 1 ppm), 72% (initial concentration 10 ppm), and 29% (initial concentration 100 ppm).
AN PAM adsorption kinetics on kaolinite are presented in Fig. 3a. For both polymers, the system reached equilibrium after about 3 h. The obtained data were better fitted to the pseudo-first-order model, than to the pseudo-second-order one. The R2 parameters for the pseudo-first-order model for AN PAM-1, and AN PAM-2 were equal to 0.9824 and 0.9459, respectively (Table 4). This indicates that polymer adsorption includes the physical interactions (hydrogen bond formation or van der Waals forces). Polyacrylamide adsorption does not meet the assumption that one macromolecule interacts with one active site, and therefore, the adsorption data obtained for the polymer were not fitted to the Langmuir and Freundlich models. A single polymer macromolecule can form ‘loops’ and ‘tails’ structures on the solid surface and, due to this fact, interact with many active sites simultaneously. The results are presented as histograms in Fig. 3b. The noticed amounts were as follows: 2.53 mg/g for AN PAM-1 and 1.71 mg/g for AN PAM-2. Thus, the larger adsorption on the kaolinite surface is observed for polyacrylamide with smaller content of ionizable carboxylic groups. The dissociation degrees (α) of used polyacrylamides at pH 5 are equal to 98% (AN PAM-1) and 98.7% (AN PAM-2). Therefore, under these conditions, almost all –COOH groups in polymer macromolecules are ionized and, as a consequence, the polymer chains are negatively charged. During the adsorption process, electrostatic repulsion between dissociated carboxylic groups (–COO−) and negative kaolinite surface ones (–SO−) occurs. This interaction is weaker for AN PAM with lower content of ionizable carboxylic groups (AN PAM-1), and as a result, the noted adsorbed amounts for this macromolecular compound are noticeably higher. In the presence of described repulsive forces, the polymer adsorption undergoes mainly through the formation of hydrogen bonds between the hydroxyl groups of kaolinite and the functional groups of polyacrylamide.
Similar measurements were carried out using the same polyacrylamides and montmorillonite (another clay mineral). At pH 5, Pb(II) ion adsorbed amount, for its initial concentration 1 ppm, was equal to 0.7 mg/g. In turn, AN PAM adsorbed amount, for its initial concentration 100 ppm, equals 127.6 mg/g (AN PAM-1) and 80.73 mg/g (AN PAM-2) (Wisniewska et al. 2018a, b). The values measured for montmorillonite were significantly higher than those for kaolinite, which was mainly dictated by larger specific surface area of montmorillonite. Gibbsite and goethite were also examined, but adsorbed polyacrylamide was characterized by different content of ionizable carboxylic groups (40%). At pH 5, AN PAM adsorbed amount measured for gibbsite, for initial polymer concentration 100 ppm, was equal to 1.76 mg/g, in turn for goethite—7.91 mg/g (Szewczuk-Karpisz et al. 2018, 2019).
Anionic polyacrylamide influence on the Pb(II) adsorption on the kaolinite surface
Anionic polyacrylamide effect on Pb(II) adsorbed amount on the kaolinite surface at pH 5 is presented in Fig. 4. The results were obtained when PAM and lead ions were added to the system simultaneously. The PAM impact on the lead adsorption process is significant for all heavy metal initial concentrations—the polymer contributes to increase in Pb(II) adsorbed amount on the mineral surface. Attractive interactions between the opposite charged lead ions and polymer macromolecules are mainly responsible for this phenomenon. Probably, during the adsorption, the polymer–metal complexes are formed that bind to the solid surface. They are relatively stable, and as a result, the heavy metal accumulation is noted. In the AN PAM-1 presence, the efficiency of lead immobilization by kaolinite (for initial ion concentration equal to 1 ppm) is about 95% (adsorbed amount equals 0.095 mg/g), whereas in the AN PAM-2 presence, it is about 96% (0.096 mg/g). When initial Pb(II) concentration is 10 ppm, the ion adsorption efficiency on the kaolinite surface is about 75% (in the AN PAM-1 presence, 0.75 mg/g) and 81% (AN PAM-2, 0.81 mg/g). In turn, when initial heavy metal concentration is 100 ppm, 74% (7.43 mg/g) and 76.5% (7.65 mg/g) of lead ions are adsorbed on the kaolinite surface in the AN PAM-1 and AN PAM-2 presence, respectively. The above observation indicates that anionic flocculant enhances the Pb(II) immobilization on the kaolinite surface (Fig. 5).
Electrokinetic properties of kaolinite particles in the absence and presence of Pb(II)/AN PAM
Electrokinetic properties of the kaolinite suspension in the absence and presence of anionic polyacrylamide/lead(II) were determined by potentiometric titration and electrophoretic mobility measurements. As can be seen in Figs. 5 and 6, both PAM and Pb(II) ions affect surface charge density (σ0) and zeta potential (ζ) of the mineral particles. In the result interpretation, absolute values of ζ and σ0 parameters were used.
In the whole studied pH range, kaolinite electrokinetic potential without additives assumes only negative values. Thus, it can be stated that the pHiep (isoelectric point) of this mineral is lower than 3. The addition of AN PAM-1 affects kaolinite zeta potential values minimally, and AN PAM-2 makes this parameter significantly higher (Fig. 5a). Kaolinite zeta potential changes after polymer addition are associated with several phenomena, inter alia, shift of the slipping plane by adsorbed AN PAM macromolecules and presence of polymer negative groups in the slipping plane area. On the kaolinite surface, the AN PAM macromolecules form ‘tails’ or ‘loops’ affecting the slipping plane position. Due to the high molecular weight of the polymer, they have a sufficient length to move this structure toward the solution and thus make the absolute value of negative zeta potential higher. AN PAM-2 contributes to more significant zeta potential changes because it contains greater number of –COO− groups in the macromolecules.
Lead(II) also changes kaolinite zeta potential values—the ζ increase was noted (the absolute value of negative zeta potential is higher) (Fig. 5b). Adsorbed Pb(II) ions are located in inner and outer Helmholtz planes (Birdi 2003). The ions adsorbed specifically (in inner Helmholtz plane) are dehydrated and not separated from the kaolinite surface by water molecules. On the other hand, Pb(II) ions adsorbed non-specifically (based on electrostatic interactions, located in outer Helmholtz plane) have hydration layers, so that they cannot approach the surface as close as the ions adsorbed specifically. After the Pb(II) addition, the heavy metal ions begin to interact with the kaolinite surface. The hydration layers of lead are more complicated and extensive than those of sodium ones (Mahler and Persson 2012; Wander and Clark 2008). As a result, their adsorption contributes to slipping plane offset and increase in absolute value of negative zeta potential.
In the suspensions containing lead(II) ions and anionic polymer simultaneously, different tendencies in zeta potential changes are observed (Fig. 5b). In the case of system with Pb(II)/AN PAM-1, the ζ changes (in comparison with the suspension without additives) are not significant. In turn, in case of system with Pb(II)/AN PAM-2, the increase in absolute values of negative zeta potential (in comparison with the system without metal ions/polymer) is clear. The AN PAM/Pb(II) complexes formed in the examined systems have a slight net charge. During their adsorption, they interact with the slipping plane structure and influence the zeta potential value.
The changes in kaolinite surface charge density (σ0) in the absence and presence of polyacrylamide/Pb(II) are presented in Fig. 6. In supporting electrolyte solution, the surface charge is formed based on the interaction between surface hydroxyl groups (–OH) and components of solution: electrolyte and hydrogen ions. The following acid–base reactions occur (Janusz 1994):
where S—the metal or semi-metal.
Moreover, the supporting electrolyte ions may form complexes with –OH groups:
The surface charge is an algebraic sum of the above group charges:
where B—the factor for the conversion of the surface concentration (μmol/m2) into charge density (μC/cm2).
Solution pH value, at which the amounts of positive and negative surface groups are identical, is called pHpzc (point of zero charge). The obtained results showed that kaolinite pHpzc parameter equals 3.37. This indicated that at pH > 3.37 the –SO− groups prevail on the soil mineral surface, whereas at pH < 3.37 the SOH2+ ones are dominant.
The anionic PAM addition makes the absolute value of negative kaolinite surface charge higher. What is more, a greater σ0 increase is observed for the polymer with higher content of dissociable functional groups (AN PAM-2) (Fig. 6a). The increase in absolute value of negative surface charge is mainly dictated by the presence of negative groups within the non-adsorbed polymer segments located slightly away from the surface. However, their amount must be greater than the number of the same functional groups attached to the surface. The negative moieties present in the adsorbed segments of the macromolecular compound contribute to decrease in absolute value of negative σ0 parameter. The experimentally observed charge on the solid surface results from the overlap of these two effects described above. The dominance of one of them determines the sign and value of solid surface charge. In the examined system, kaolinite is negatively charged, and due to this fact, a lot of negative functional groups are located in the non-adsorbed polymer segments. The number of these groups is prevailing and therefore an increase in the absolute value of negative surface charge is noted.
The lead addition (without the polymer) also changes the kaolinite surface charge density (Fig. 6b). There is a slight increase in the absolute value of negative σ0 parameter. This phenomenon is particularly due to the presence of lead(II) ions in the inner Helmholtz plane, which predominate quantitatively over the lead(II) ions present in the outer Helmholtz plane.
In the systems containing AN PAM/Pb(II), a clear decrease in absolute value of negative solid surface charge density compared to the system without additives was observed (Fig. 6b). What is more, the results obtained for AN PAM-1 and AN PAM-2 were similar. The AN PAM/Pb(II) complexes assume such a conformation at the kaolinite/aqueous solution interface that dissociated functional groups are located very close to the solid and contribute to significant σ0 decrease.
Lead and polyacrylamide impact on the kaolinite aggregation
Figure 7 shows the stability changes in time for the kaolinite suspension in the absence and presence of polyacrylamide/Pb(II) ions. The system without any additives is characterized by high initial absorbance equal to 2.7, which gradually decreases over time. This indicates that the mutual contact between particles is difficult and, as a result, the aggregate formation is very slow. The AN PAM adsorption contributes to different changes in the suspension stability, depending on ionizable group content. AN PAM-1 causes a slight reduction in the system stability—the absorbance decrease recorded for the system is faster (Fig. 7a). This is probably associated with polymer bridges formation by adsorbed macromolecules that promote aggregation. A single bridge is created by one AN PAM macromolecule adsorbed on at least two solid particles. This phenomenon is very probable when: (1) solid surface is incompletely coated with the polymer macromolecules and (2) macromolecular compound is characterized by high molecular weight (then the length of polymer chains is sufficient for simultaneous binding to two or more solid particles) (Birdi 2003). On the other hand, AN PAM-2 causes a slight increase in the suspension stability—after 60 min the absorbance recorded for the suspension containing AN PAM-2 was higher than that noted without the polymer. AN PAM-2 macromolecules contain higher number of dissociated carboxylic groups that repel each other more strongly. Consequently, the polymer bridge formation and kaolinite aggregation are clearly limited (Fig. 7b).
The adsorption of AN PAM/Pb(II) complexes on the kaolinite surface contributes mainly to the suspension stability increase. As it was mentioned above, the adsorbed structures have a slight charge and, due to this fact, the formed adsorption layers repel each other electrostatically. Based on these results, it can be stated that environmental pollution with lead limited the flocculation ability of polyacrylamide. This Pb(II) impact is especially noted for AN PAM-1, which stimulates the kaolinite aggregation in the environment without heavy metal.
Conclusion
At pH 5, both AN PAM-1 and AN PAM-2 adsorb on the kaolinite surface, but higher adsorbed amount is noted for AN PAM-1 due to weaker electrostatic repulsion occurring between negative polymer macromolecules and negative kaolinite surface. Lead ions have a strong affinity to the mineral surface, but PAM addition makes the Pb(II) adsorbed amount even higher. During the adsorption there is a complex formation between Pb(II) and PAM macromolecules occurring mainly by electrostatic interactions. As a result in the polyacrylamide presence, the lead immobilization on the kaolinite surface is more effective. Adsorption of anionic polyacrylamide and lead(II) ions affects the surface electrokinetic properties of kaolinite. The changes in the particle surface charge density (σ0) as well as zeta potential (ζ) are observed. The Pb(II) presence affects also the aggregation of kaolinite particles. In case of AN PAM-1 after the lead addition, a strong limitation in the polymer flocculation ability was noted.
References
Alkorta I, Hernandex-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Rev Environ Sci Biotech 3(1):71–90
Anovitz LM, Cole DR (2015) Characterization and analysis of porosity and pore structure. Rev Mineral Geochem 80:161–164
Birdi KS (2003) Handbook of surface and colloid chemistry, 2nd edn. CRS Press, Boca Raton
Crummett WB, Hummel RA (1963) The determination of traces of polyacrylamides in water. J Am Water Works Assoc 1:209–219
Dagnall RM, West TS, Young P (1965) Determination of lead with 4-(2-pyridylazo)-resorcinol II: application to steel, brass and bronze. Talanta 12:583–588
Fahmi AH, Samsuri AW, Jol H, Singh D (2018) Bioavailability and leaching of Cd and Pb from contaminated soil amended with different sizes of biochar. R Soc Open Sci 5(11):181328
Gadepalle VP, Ouki SK, Van Herwijnen R, Hutchings T (2007) Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. Soil Sediment Contam 16:233–251
Grządka E, Matusiak J (2017) The effect of ionic and non-ionic surfactants and pH on the stability, adsorption and electrokinetic properties of the alginic acid/alumina system. Carbohydr Polym 175:192–198
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ho YS, McKay G (2002) Application of kinetics models to the sorption of copper(II) onto peat. Adsorpt Sci Technol 20:797–815
Houben D, Picar J, Sonnet P (2012) Heavy metal immobilization by cost-effective amendments in a contaminated soil: effects on metal leaching and phytoavailability. J Geochem Explor 123:87–94
Hunter RJ (1981) Zeta potential in colloid science. Academic Press, New York
Janusz W (1994) Electrical double layer at the metal oxide/electrolyte interface in interfacial forces and fields: theory and applications. In: Decker M (ed) Surfactant science, chapter 4, vol 85. Dekker Inc, New York
Kielcew NW (1980) Podstawy techniki adsorpcyjnej. Wydawnictwo WNT, Warszawa
Lwin CS, Seo BH, Kim HU, Owens G, Kim KR (2018) Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—a critical review. Soil Sci Plant Nutr 64(2):156–167
Mahler J, Persson I (2012) A study of the hydration of the alkali metal ions in aqueous solution. Inorg Chem 51:425–438
Mahmoud EK, El-Kader NA (2013) Soil immobilization of heavy metal using soil amendments in a greenhouse study. Compos Sci Util 21(3/4):156–163
Mahmoud EK, El-Kader NA (2015) Heavy metal immobilization in contaminated soils using phosphogypsum and rice straw compost. Land Degrad Dev 26(8):819–824
Ndi KS, Kofa GP, Metiotsop TD, Amba EV, Kayem GJ (2015) Kinetics and equilibrium studies of fluoride adsorption on laterite and kaolinite: comparative studies. J Appl Res 1(8):269–276
Niesiobędzka K (2003) The effect of heavy metal leaching from plant surface by rainwater (in Polish). Chemia i Inżynieria Ekologiczna 10(2):302–312
Numbonui Ghogomu J, Tsemo Noufame D, Buleng Njoyim Tamungang E, Ajifack DL, Nsami Ndi J, Mbadcam J (2014) Adsorption of phenol of aqueous solutions onto natural and thermally-modified kaolinitic materials. Int J Biol Chem Sci 8(5):2325–2338
Ociepa-Kubicka A, Ociepa E (2012) Toxic effects of heavy metals on plants, animals and humans (in Polish). Inżynieria i Ochrona Środowiska 15:169–180
Oliveira WE, Franca AS, Oliveira LS, Rocha SD (2008) Untreated coffee husks as biosorbent for the removal of heavy metals from aqueous solutions. J Hazard Mater 152:1073–1081
Ościk J (1969) Adsorpcja. Wydawnictwo UMCS, Lublin
Oste LA, Lexmond TM, Van Riemskijk WH (2002) Metal immobilization in soils using synthetic zeolites. J Environ Qual 31(3):813–821
Paderewski ML (1999) Procesy adsorpcyjne w inżynierii chemicznej. Wydawnictwo WNT, Warszawa
Szewczuk-Karpisz K, Krasucka P, Boguta P, Skic K, Sokołowska Z, Fijałkowska G, Wiśniewska M (2018) Electrical double layer at the gibbsite/anionic polyacrylamide/supporting electrolyte interface—adsorption, spectroscopy and electrokinetic studies. J Mol Liq 261:439–445
Szewczuk-Karpisz K, Krasucka P, Boguta P, Skic K, Sokołowska Z, Fijałkowska G, Wiśniewska M (2019) Anionic polyacrylamide efficiency in goethite removal from aqueous solutions: goethite suspension destabilization by PAM. Int J Environ Sci Technol 16(7):3145–3154
Szymański K (2009) The lead and chromium compounds in natural environment and wastes (in Polish). Rocznik Ochrona Środowiska 11:173–181
Tomczyk A, Boguta P, Sokołowska Z (2019) Biochar efficiency in copper removal from Haplic soils. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02227-4
Wander MCF, Clark AE (2008) Hydration properties of aqueous Pb(II) ion. Inorg Chem 47(18):8233–8241
Wang H, Zhou A, Peng F, Yu H, Yang J (2007) Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II). J Colloid Interface Sci 316:277–283
Wang W, Liu P, Zhang M, Hu J, Xing F (2012) The pore structure of phosphoaluminate cement. Open J Compos Mater 2:104–112
Wang T, Sun H, Ren X, Li B, Mao H (2017) Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci Rep 7:12114
Wiśniewska M, Fijałkowska G, Szewczuk-Karpisz K (2018a) The mechanism of anionic polyacrylamide adsorption on montmorillonite surface in the presence of Cr(VI) ions. Chemosphere 211:524–534
Wiśniewska M, Fijałkowska G, Szewczuk-Karpisz K, Urban T, Nosal-Wiercińska A, Wójcik G (2018b) Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium(VI) ions. Adsorption. https://doi.org/10.1007/s10450-018-9990-x
Xu Y, Liang X, Xu Y, Qin X, Huang Q, Wang L, Sun Y (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27(2):193–204
Zhang Z, Xia S, Wang X, Yang A, Xu B, Chen L, Zhu Z, Zhao J, Jaffrezic-Renault N, Leonard D (2009) A novel biosorbent for dye removal: extracellular polymeric substance of Proteus mirabilis TJ-1. J Hazard Mater 163(1):279–284
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fijałkowska, G., Szewczuk-Karpisz, K. & Wiśniewska, M. Anionic polyacrylamide as a substance strengthening the Pb(II) immobilization on the kaolinite surface. Int. J. Environ. Sci. Technol. 17, 1101–1112 (2020). https://doi.org/10.1007/s13762-019-02546-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02546-6