Abstract
Cenospheres, which are the by-product of hard coal combustion, are characterized by properties allowing for a broad use of this material. The article presents the results of the chemical composition analysis of the cenospheres obtained from various power plants. It has been exhibited that depending on the place of origin, their chemical composition is similar and comparable to fly ash produced in hard coal combustion. A great majority of analysed cenospheres may be classified similarly as the sialic ashes—slightly acidic. The analysis of dependence of refractoriness of the cenospheres on their chemical composition confirmed the correlation between this parameter and the content of SiO2 and Al2O3. At the same time, the content of each of the chemical components indicates a correlation with the size of the cenospheres. A trend of SiO2/Al2O3 ratio decreasing along with the increasing size of the cenospheres has been noted. Also the content of K2O and—to a lesser extent—Na2O decreases along with increasing diameter of grains. An inverse correlation was noted in case of Fe2O3. The higher the diameter of the grains, the higher the content of this component. In view of the obtained results, it should be assumed that the division of cenospheres into grain-size classes before further industrial use could extent the current range of use of this material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cenospheres are a by-product, but also one of the more valuable coal combustion products (Ranjba and Kuenze 2017). Most often, cenospheres are spherical and filled with gas (mostly CO2 and N2) (Li 2012; Pichór 2005; Pichór and Petri 2003; Acar and Atalay 2016; Novoselova et al. 2008). Cenospheres are particles of ash with a density lower than the density of water, which facilitates their separation by floatation in sedimentation tanks of power plants (Manocha et al. 2011). Their size usually fits within the range from 5 to 500 μm, usually from 20 to 200 μm (Pichór 2005; Joseph et al. 2013; Soh et al. 2016). Sporadically, also larger particles have been noted in some ashes (Vassilev et al. 2004; Żyrkowski et al. 2016; Strzałkowska and Stanienda Pilecki 2018). The thickness of the walls of cenospheres usually constitutes from 5 to 10% of their diameter (Pichór and Petri 2003; Strzałkowska and Stanienda Pilecki 2018). One of the key parameters determining the possible use of waste is the chemical composition (Uliasz-Bocheńczyk and Mokrzycki 2018). The largest part is constituted by SiO2 silica (45–60%) and Al2O3 white clay (20–30%) (Żyrkowski et al. 2016; Haustein and Quant 2011; Kapuściński and Strzałkowska 2008; Senthamarai Kannan et al. 2016; Fomenko et al. 2012), which ensures their high mechanical strength (Żyrkowski et al. 2016; Dey and Pandey 2016). The remaining components: Fe2O3 and CaO, MgO, SO3, K2O, Na2O and TiO2, occur in low concentrations (Vassilev et al. 2004; Strzałkowska 2017; Ngu et al. 2007). The chemical composition of cenospheres is determined by the type of the mineral substance present in coal, which is a subject to transformations related to high temperature: dehydration, decarbonatization or oxidation. Reduction in iron oxides is considered one of the most important reactions in the process of mineral substance expansion, as it reinforces the processes of coal oxidation and combustion. According to Raask (1968), the content of iron oxides is an important factor determining the formation of microspheres. The authors of the work (Ngu et al. 2007; Yu et al. 2012) do not share this opinion, stating that iron oxide is the main component of the magnetic fraction of ashes but is not necessary in the formation of cenospheres. The presence of Fe2O3 and TiO2, on the other hand, causes a change of colour of cenospheres from white to yellow or brown (Żyrkowski et al. 2016).
Authors of the works (Żyrkowski et al. 2016; Itskos 2010) provide that a correlation exists between the content of Na2O and CaO and the amount of cenospheres obtained from fly ash. The increase in sodium content—at a simultaneously decreased content of calcium, facilitates the intensification of the cenosphere formation in ash. According to the authors, a larger amount of calcium causes a lower viscosity, which is adverse in terms of the glass formation process. The positive impact of sodium in the cenosphere formation process is, on the other hand, debatable. One possible explanation is the fact that sodium may be supported by NaCl and the chlorine itself may increase the viscosity of alloy in certain conditions (Vassilev et al. 2004; Żyrkowski et al. 2016). According to Łączny and Wałek (2011), the amount of cenospheres obtained from fly ash results mostly from the parameters of combustion process and not the composition of the feed material. The dependence of the chemical composition and the size of microspheres is rarely mentioned. It has been observed that the high value of the SiO2/Al2O3 indicator facilitates the formation of particles with a smaller diameter (Haustein and Quant 2011; Ngu et al. 2007), while the content of iron oxide, magnesium and sodium decreases along with the increasing cenosphere diameters (Haustein and Quant 2011). The earlier findings by Itskos (2010) have not been thus confirmed, as he observed that larger particles contain more sodium and magnesium oxides and less calcium oxide. The literature also notes the fact that the concentrations of Cu, Zn, Pb, Ni and Cr are increased along with increasing particle diameters (Haustein and Quant 2011; Bradło 2016). This dependence was not confirmed for cadmium (Haustein and Quant 2011). Although the content of the elements in cenospheres is highly variable, the highest concentrations were exhibited by alkaline metals, mostly Ba, Sr and Rb (Bradło 2016).
The purpose of this study was to determine the extent to which the size of the cenospheres is related to their chemical composition. The study encompassed samples of cenospheres available at the domestic and foreign markets.
Materials and methods
For the purpose of the study, samples of microspheres from sedimentation tanks of three power plants were acquired (Dolna Odra, Opole and Kazakhstan).
The granulometric composition of the acquired samples was established using the sieve method. In the tests, the following mesh sizes were used: 0.8; 0.7; 0.6; 0.5; 0.25; 0.125; 0.071; 0.063; and 0.045 mm.
The chemical composition was established by means of X-ray fluorescence method using the ZSX PRIMUS spectrometer manufactured by RIGAKU.
The study was extended to microanalyses using a Hitachi SEM SU3500 vacuum microscope with variable scanning microscope, cooperating with an X-ray spectrometer with EDD UltraDry energy dispenser from the Thermo Scientific NORAN 7 system. X-ray microanalysis was carried out using the following parameters: accelerating voltage—15 keV, working distance (WD)—10 mm, pressure—30 Pa, vacuum—variable. Four grain classes of cenospheres were selected for the tests, namely < 0.045 mm; 0.045–0.063 mm; 0.125–0.25 mm; and 0.25–0.50 mm.
Results and discussion
Granulometric composition of the cenospheres
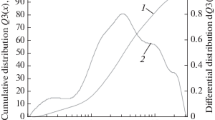
Nine grain classes were distinguished in granulometric analysis. Based on the calculated percentage of each of the grain classes, cumulative curves of size composition were prepared (Fig. 1) The largest grain sizes were exhibited by the sample of cenospheres from Kazakhstan. The class exceeding 0.125 mm constituted as much as 95% of the sample, while it was only 23% of the sample acquired from the Dolna Odra plant. The location of the cumulative curve, which represents a sample of microspheres from the Dolna Odra power plant, near the left-bottom corner of the graph, confirms the high content of fine grains below 0.3 mm (almost 100%) in the material.
Chemical composition of cenospheres (XRF)
In the microspheres from Opole, Dolna Odra and Kazahstan power plants, SiO2 and Al2O3 were the dominant chemical components. Their total content exceeded 80% of the mass and reached as much as 90% of the mass in case of the power plant in Kazakhstan (Table 1). In case of the latter, characterized by the largest grains, the value of the SiO2 to Al2O3 indicator was the lowest. The content of components, such as TiO2, Fe2O3, MgO, CaO and K2O, reached only several per cent in all the samples, while the content of remaining components has not reached 1% of the mass. The chemical composition of cenospheres which were a subject to the study was similar to the composition of fly ash (Adamczyk and Białecka 2005; Kurdowski 2010; Haustein and Quant 2011; Giergiczny et al. 2016).
The sample of the cenospheres from the power plant in Kazakhstan, characterized by the highest total content of silica and white clay (over 90% of mass), and the low content of fluxes (approx. 6%), has exhibited the highest refractoriness, that is, 1685 °C. In case of the sample of cenospheres from the Opole Power Plant, that is, the one with the lowest refractoriness, the lowest content of Al2O3 was also noted, while the total of oxides classified as fluxes exceeded 14% of the sample’s mass.
Microanalysis of the chemical composition of cenospheres (SEM)
To make the results more precise, the study was complemented with a chemical microanalysis of the selected grain-size classes of the cenospheres. The results are listed in Table 2 and presented in Figs. 2, 3, 4, 5, 6, 7 and 8. The performed analyses have indicated local variability of the chemical composition of cenospheres, while the following has been noted: increase in the Fe2O3, MgO and CaO contents along with the increase in the size of the cenospheres (Fig. 2, 3) and decreasing trend of K2O content along with the increasing size of cenospheres.
Position of the Opole Power Plant cenospheres in the classification of fly ash produced in coal combustion (Vassilev and Vassileva 2007)
Position of the Dolna Odra plant cenospheres in the classification of fly ash produced in coal combustion (Vassilev and Vassileva 2007)
Position of the Kazakhstan power plant cenospheres in the classification of fly ash produced in coal combustion (Vassilev and Vassileva 2007)
A similar dependence as K2O was observed for Na2O, but it was not that strong (Fig. 3).
Higher contents of alkalis (K2O and Na2O) in smaller-size microspheres are probably related to their melting point. As is well known, K2O and Na2O lower the melting point; components such as Fe2O3 and MgO lower it slightly, whereas SiO2 and Al2O3 increase its value (Vassilev et al. 1995; Jing et al. 2012; Drzymała et al. 2014; Mishra et al. 2016). Therefore, mineral components containing alkalis will melt faster than the components without them. Drops of an alloy with a higher content of K2O and Na2O, in the stream of flue gas, will be characterized by lower viscosity than a drop of an alloy with a smaller amount of these components. Due to the fact that all solid and liquid components (alloy drops) contained in the flue gas stream are in motion, they must collide with each other. An alloy drop after hitting a solid grain may: (1) splash (very low viscosity of the alloy—higher contents of K2O and Na2O), (2) spread on its surface (higher viscosity of the alloy—low contents of K2O and Na2O) or (3) adhere to it (the highest viscosity of the alloy—the lowest contents of K2O and Na2O), forming aggregates (Fig. 8d). In the first case, they will form cenospheres with the smallest dimensions, which will grow as the contents of K2O and Na2O decrease.
At the same time, a considerable decrease in the value of the SiO2/Al2O3 ratio has been noted along with the increase in the size of cenospheres (Fig. 4).
The projection of chemical composition of the cenospheres (constituting a prepared part of fly ash) in a classification triangle presented in Vassilev and Vassileva (2007) indicates that a vast majority of tested cenospheres is classified similarly to sialic ashes, irrespective of their place of origin (power plant) (Figs. 5, 6, 7). The remaining cenospheres are also of different types, mostly ferrisialic and rarely calsialic.
Conclusion
The presented results of the study have exhibited that cenospheres originating in different power plants may vary considerably in terms of grain sizes. Irrespective of the place of origin, however, their chemical composition is similar and comparable to fly ash produced in hard coal combustion. The analysis of dependence of refractoriness of cenospheres on their chemical composition has confirmed a positive correlation between the refractoriness of a sample and the content of SiO2 and Al2O3. The projection of chemical composition in the classification triangle indicates that most of the analysed cenospheres may be classified as sialic ashes, or—least frequently—ferricalsialic and calsialic. At the same time, the content of individual chemical components is correlated to the size of the cenospheres. A trend of SiO2/Al2O3 ratio decreasing along with the increasing size of the cenospheres has been noted, which had been indicated in the professional literature earlier. The increase in the diameter of the cenospheres was simultaneously related to the increase in the iron content. It may be thus assumed that the presence of iron oxides facilitates the formation of larger cenospheres, but is not necessary in their formation. The formation of larger diameters of the particles is dependent on gases causing an outward pressure in the particle. According to Raask (1968), this requires a certain amount of iron oxide. In wet separation of cenospheres, the larger particles will float more easily in the sedimentation tank, because the gravity force exerted on the cenosphere will have a smaller value than the buoyant force. A similar correlation between the increase in the content of a component and the increasing diameter of the particles has been noted for magnesium oxide and—to a lesser extent—for calcium oxide. The correlation previously provided in the professional literature has not been thus confirmed (Itskos 2010). A detailed interpretation of SEM (scanning electron microscope) results allows for a partial explanation of this phenomenon. The coarser class of grains often contains particles with much lower sphericity and inclusions of finer particles (Fig. 8). These grains might disturb the dependencies found earlier. It should be noted that the study by Itskos did not concern cenospheres, but ashes produced in the combustion of brown coal. It is common knowledge that ashes of that type exhibit a high content of calcium oxides.
K2O and Na2O are important chemical components of cenospheres, because as their content increases, the diameter of cenospheres decreases. The mechanism of the formation of cenospheres with different contents of these components is quite complicated and remains correlated with the temperature and viscosity of molten mineral components.
The conducted study has confirmed that size of cenospheres is related to their chemical composition. In view of the obtained results, it should be assumed that the division of cenospheres into grain-size classes before their further industrial use could extent the current range of use of the material. Both chemical composition and sizes of cenospheres are main parameters that are decisive for their further use. A continuous control over these parameters may allow to obtain a product characterized by a higher quality. Due to the high demand, also the cost of production is of significance.
References
Acar I, Atalay MU (2016) Recovery potentials of cenospheres from bituminous fly ashes. Fuel 180:97–105. https://doi.org/10.1016/j.fuel.2016.04.013
Adamczyk Z, Białecka B (2005) Hydrothermal synthesis of zeolites from Polish coal fly ash. Pol J Environ 14(6):713–719
Bradło D (2016) Modified aluminasilicate microspheres as a filler in polymer composites. Dissertation, Cracow University of Technology, Kraków
Dey A, Pandey KM (2016) Characterization of fly ash and its reinforcement effect on metal matrix composites: a review. Rev Adv Mater Sci 44:168–181
Drzymała P, Długosz P, Darłak P, Sobczak JJ (2014) Fly ash as a reinforcement of light metal matrix composites part 3. The influence of a chemical composition on characteristic fusibility temperatures of coal fly ashes. Trans Foundry Res Inst 54(2):57–72. https://doi.org/10.7356/iod.2014.08
Fomenko EV, Anshits NN, Pankova MV, Mikhaylova OA, Solovyov LA, Shishkina NN, Anshits AG (2012) Influence of the composition and structure of the glass crystalline shell of cenospheres on helium permeability. Glass Phys Chem 38(2):218–227
Giergiczny Z, Ostrowski M, Baran T (2016) Wpływ popiołów lotnych krzemionkowych kategorii S na wybrane właściwości kompozytów cementowych. Międzynarodowa Konferencja Popioły z Energetyki–Zakopane 19–21.X.2016 r: 1–17
Haustein E, Quant B (2011) The characteristics of selected properties of the cenospheres—fraction of fly ash—by-product of coal combustion. Gospod Surowcami Mineral Mineral Resour Manag 27(3):95–111
Itskos G (2010) Size fraction characterization of highly-calcareous fly ash. Fuel Process Technol 91:1558–1563. https://doi.org/10.1016/j.fuproc.2010.06.002
Jing N, Wang Q, Yang Y, Cheng L, Luo Z, Cen K (2012) Influence of ash composition on the sintering behavior during pressurized combustion and gasification process. J Zhejiang Univ Sci A 13:230. https://doi.org/10.1631/jzus.A1100206
Joseph KV, Finjin F, Joyson Ch, Das P, Hebbar G (2013) Fly ash cenosphere waste formation in coal fired power plants and its applicationas a structural material—a review. IJERT 2(8):1236–1260
Kapuściński T, Strzałkowska E (2008) Cenospheres from fly ashes of Łaziska Power Plant. Mineral Special Pap 32:83
Kurdowski W (2010) Chemia cementu i betonu. Wyd. Polski Cement/Wyd. Naukowe PWN, Kraków (in Polish)
Łączny MJ, Wałek T (2011) Modelowanie procesu powstawania cenosfer w kotłach pyłowych. Popioły z energetyki, Zakopane, pp 191–203
Li Y (2012) Ash cenosphere formation, fragmentation and its contribution to particulate matter emission during solid fuels combustion. PhD thesis, Curtin University
Manocha LM, Ram KA, Manocha SM (2011) Separation of cenospheres from fly ashes by floatation method. Eurasian Chem Tech J 13:89–95
Mishra V, Bhowmick T, Chakravarty S, Varma AK, Sharma M (2016) Influence of coal quality on combustion behaviour and mineral phases transformations. Fuel 186:443–455. https://doi.org/10.1016/j.fuel.2016.08.092
Ngu L, Wu H, Zhang D (2007) Characterization of ash cenospheres in fly ash from Australian power station. Energy Fuels 21(6):3437–3445. https://doi.org/10.1021/ef700340k
Novoselova LYu, Sirotkina EE, Pogadaeva NI, Russkikh IV (2008) Aluminosilicate microspheres in fly ashes from thermal power plants and their use for the removal of petroleum and phenol from water. Solid Fuel Chem 42:177–182
Pichór W (2005) The direction of possible applications of the cenospheres from coal ash in civil engineering. Mater Ceram 4:160–165
Pichór W, Petri M (2003) Właściwości mikrosfer pozyskiwanych jako uboczny produkt spalania węgla kamiennego. Ceramika 80:705–710
Raask E (1968) Cenospheres in pulverized-fuel ash. J Inst Fuel 41:339–344
Ranjba N, Kuenze C (2017) Cenospheres: a review. Fuel 207:1–12. https://doi.org/10.1016/j.fuel.2017.06.059
Senthamarai Kannan K, Andal L, Shanmugasundaram M (2016) An investigation on strength development of cement with cenosphere and silica fume as pozzolanic replacement. Adv Mater Sci Eng 1:1–5. https://doi.org/10.1155/2016/9367619
Soh WM, Tan J, Heng JY, Cheeseman CR (2016) Production of cenospheres from coal fly ash through vertical thermal flame (VTF) process. Mater Sci Forum 880:7–10
Strzałkowska E (2017) Selected properties of cenospheres from fly ashes. Przegląd Górniczy 73(11):37–43
Strzałkowska E, Stanienda Pilecki K (2018) Morphology, size and density of cenospheres from Polish and foreign power plants. Przegląd Górniczy 74(12):44–53
Uliasz-Bocheńczyk A, Mokrzycki E (2018) The elemental composition of biomass ashes as a preliminary assessment of the recovery potential. Gospod Surowcami Mineral Mineral Resour Manag 34(4):115–132
Vassilev SV, Vassileva CG (2007) A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behavior. Fuel 86:1490–1512. https://doi.org/10.1016/j.fuel.2006.11.020
Vassilev SV, Kitano K, Takeda S, Tsurue T (1995) Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Process Technol 45:27–52
Vassilev SV, Menendez R, Borrego AG, Diaz-Somoano M, Martinez-Tarazona MR (2004) Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 83(11–12):1563–1583. https://doi.org/10.1016/j.fuel.2004.01.010
Yu J, Li X, Fleming D, Meng Z, Wang D, Tahmasebi A (2012) Analysis on characteristics of fly ash from coal fired power stations. Energy Proc 17:3–9. https://doi.org/10.1016/j.egypro.2012.02.054
Żyrkowski M, Neto RC, Santos LF, Witkowski K (2016) Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 174:49–53. https://doi.org/10.1016/j.fuel.2016.01.061
Acknowledgements
The article has been written as a result of a research project financed by Silesian University of Technology, Faculty of Mining and Geology, from the funds of the Ministry of Science and Higher Education [Grant No. BK nr 06/060/BK 17/0035 (BK261/RG6/2017)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Strzałkowska, E., Adamczyk, Z. Influence of chemical composition of fly-ash cenospheres on their grains size. Int. J. Environ. Sci. Technol. 17, 809–818 (2020). https://doi.org/10.1007/s13762-019-02512-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02512-2