Abstract
This research shows a method of elimination of methylene blue from aqueous solutions using raphia fiber. Sorption was applied in a bath system and in the system with mixing and online measurement. The obtained data in equilibrium can suggest adjusting the sorption isotherm model and calculation of the maximum sorption capacities. The Langmuir isotherm model has the highest correlation coefficient R2 which shows that the adsorption process of methylene blue on the raphia fibers is a monolayer adsorption process. On the other hand, the Freundlich isotherm is also well fitted, which may indicate that the isotherm is also well adapted, while in the system with mixing and online measurement, the highest matching factor is characterized by the Freundlich isotherm model. The pseudo-second-order rate model is best fit for the sorption kinetics, on basis of which it can be concluded that the sorption rate-limiting step is chemisorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyes belong to one of the most popular impurities occurring in wastewater, obtained in industrial processes (dyeing of materials) (Adeleke et al. 2018). On average, 13% of the produced dyes in the world after the dyeing process are discharged to sewage (Sun et al. 2011; Safavi and Momeni 2012). The danger of dye processing is the possibility of their transformation into toxic or carcinogenic compounds. Depending on the concentration of dyes, their negative impact contributes to the degradation of the environment and the deterioration of human life. In addition, dyes may also contribute to the disruption of water management, because the color substances can prevent the penetration of light into water (Rane et al. 2014; Khanday et al. 2017).
Methylene blue is included in the example of dyes widely used in the textile industry. Exposure of people to methylene blue can cause negative health effects such as shock, vomiting, jaundice, cyanosis, and tissue necrosis. In view of the above, it seems necessary to find new ways to detoxify industrial wastewater with methylene blue (Ghosh and Bhattacharyya 2002; Hameed et al. 2007; Rahmi and Mustafa 2019).
The problem in removing dyes results from the inability to completely remove them using standard biological processes among which non-alcoholic fermentation can be distinguished. By using different cultures of bacteria and fungi in aerobic and anaerobic conditions, decolourization attempts have been made. The problem of the use of decolourization under anaerobic conditions is associated with the formation of toxic aromatic amines, while under aerobic conditions, total mineralization occurs (Nigam et al. 2000). Up to 90% of most toxic dyes are unmodified after treatment by activated sludge. Sewage produced by the textile industry has an intense color and high concentrations of organic and inorganic substances dissolved in them. In the work of Mouni et al. (2018), the authors tested several different technologies enabling modification and removal of harmful dyes, which include flocculation combined with flotation, electroflotation ozonation, membrane separation, chemical oxidation, electrochemical destruction, electrochemical techniques, adsorption, or photocatalysis (Mouni et al. 2018; Nataraj et al. 2009).
Currently, adsorption due to its high efficiency, easy availability, and low price is increasingly used to remove dyes from aqueous solutions. Therefore, many scientific works present the results of removing processed dyes from sewage using cheap adsorbents, e.g., natural minerals or biological species, agricultural by-products, wastes from various industrial processes (De Gisi et al. 2016; Bhatnagar et al. 2015; Ramesh et al. 2007; Pal et al. 2018).
The removal of methylene blue from the model solutions was the purpose of this work. Raphia farinifera fibers as a biosorbent were used for the sorption process. The used fiber belongs to the group of organic materials, which in its composition include lignin and cellulose (Elenga et al. 2009). Sorption process tests were carried out in the “bath”-type system and in a mixed system with simultaneous online measurement of the dye concentration. Testing the sorption process in the “bath” system allows obtaining information on the maximum sorption capacity of raphia fibers in relation to methylene blue, while the system with mixing and online measurement allows obtaining information on simulation of conditions on a scale larger than the laboratory scale, and continuous measurement is a response to the demand for environmentally friendly technologies in which only continuous control of process parameters allows reliable assessment of the effectiveness of various solutions for environmental protection. The use of the system with mixing and the return of the solution significantly affect the kinetics (diffusion parameters). The research allowed the determination of sorption capacity of raphia fibers in relation to methylene blue, adjustment of the appropriate sorption model, and determination of sorption kinetics for both systems. Calculated parameters of the sorption process for the “bath” system and online measurement allowed to obtain basic information about the mechanisms of the sorption process.
Materials and methods
Materials
The research involved the use of raphia fibers purchased in the commercial shop. The raphia fibers before the tests were washed with deionized water until the supernatant after washing was clear. All dye solutions were prepared in distilled water. Reagents used in the tests were purchased from Sigma-Aldrich and were characterized by analytical purity.
Sorption process
Sorption in the “bath” type
The sorption of methylene blue was carried out in a dynamic type system called “bath.” The regulated process parameters were the concentration of methylene blue and the time of the sorption process.
The sorption process was carried out in 60-cm3 beakers. About 0.2 g of sorbent was used for each test. A 40 cm3 solution containing methylene blue of various concentrations: 100, 150, 200, 250, and 300 mg/dm3, was added to the prepared set. The dye sorption process was carried out for 5, 10, 20, 30, 45, and 60 min. After the end of the process, the solution was filtered and analyzed spectrophotometrically (Rayleigh UV-1800). The sorption process was repeated three times, and the results obtained were averaged.
Sorption in the tank with mixing and online measurement
The online dye sorption process was carried out in a closed system. The set consisted of an 800-cm3 beaker, a peristaltic pump, a flow cell, and a spectrophotometer. Into the beaker, 500 cm3 of methylene blue solution at a concentration of 11 mg/dm3 (maximum concentration measurable online) was added then followed by 1.0, 1.25, 1.50, 1.75, and 2.0 g of raphia. Differences in the concentrations used between the two systems result from hardware limitations (it is not possible to test solutions in online mode above 11 mg/dm3), and only such a system allows testing of the continuous sorption process. Measurements of dye concentration change were carried out for 90 min every 5 s. The sorption process was repeated three times, and the results obtained were averaged.
Sorption equilibrium
The formulas used to calculate the sorption capacity at any time (qt mg/g) and at equilibrium (qe mg/g), and the percentage removal at the equilibrium (Re %) were used to calculate the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherms.
Sorption kinetics
In order to understand the dynamics of methylene blue sorption on the raphia fibers in relation to the time of conducting the process, three kinetic models shown in Table 1 were used. For the “bath” system, different initial concentrations (100–300 mg/dm3) were used at constant mass. In mixing and online measurement, different mass (0.5–1.5 g) at a constant concentration to determine the effect of changing parameters on the sorption process was used.
Results and discussion
Sorbent characterization
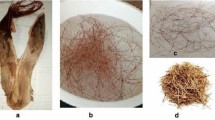
SEM micrographs (Hitachi TM-3000 equipped with an X-ray microanalyzer EDS) of the raphia fibers before and after the process of adsorption are shown in Fig. 1. It can be seen that the raphia is characterized by a heterogeneous, fibrous nature, and it can be clearly seen that these sample indicate well-defined mesoporous structure (Fig. 2). In order to check the surface area of raphia fibers, the Macrometrics camera capable of measuring the micropores with ASAP 2010 degassing station had been used. The samples before the measurements were dried in atmosphere of helium at 110 °C during 8 h and in the next step under vacuum at 100 °C and 0.001 Tor for 8 h. The analysis of the fibers surface was performed with an LEO 1430 VP scanning electron microscope. The results of surface area of the adsorbent were equal to 1.08 m2/g.
On the micrographs shown on Fig. 3, it can be seen that the surface of the adsorbent after the sorption process is covered by nitrogen from methylene blue. The fiber’s surfaces appear to be covered by methylene blue, attesting the strong adhesion between the composite components.
In order to check the ratio of C/H/N, the analysis with the use of the CHN analyzer (Perkin Elmer Type 2400) was carried out. The results showed that the content of elements were equal to: C—46.04%, H—6.18%, and N—0.77%. The contents of C, H, and N in raphia are similar to the content of these elements in materials of organic origin (Bispo et al. 2018; Chwastowski et al. 2017). The presence of these elements is also observed in the FTIR analysis.
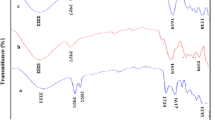
Fourier transform infrared spectroscopy (Scimitar Series FTS 2000 from Digilab) spectra of the raphia fibers before and after the sorption process are shown in Fig. 4. The peak at 1030 cm−1 corresponds to C–OH stretching, while the peak at 1640 cm−1 is consistent with the presence of carbonyl groups (COO), and a peak at 2917 cm−1 corresponds to the presence of CH2 (asymmetric stretch). The other absorption occurring at 1238 cm−1, 1729 cm−1, 1735 cm−1, and 3302 cm−1 corresponds to C–N stretching vibrations, C=O of amide, C–H stretching vibrations, CH3 bending, and O–H of alcohol, respectively (Inyinbor et al. 2016). On raphia fibers after sorption of methylene blue, one could observe compounds exhibited the dominant peak corresponding to the C–C stretching of methylene blue at 1600 cm−1 (Kumpan et al. 2017).
Influence of the contact time, the initial concentration and mass
“Bath”-type sorption process
Sorption capacity of raphia fibers is dependent on the time of the process and the initial concentration of used methylene blue which is shown in Fig. 5. Very high removal of dye at the used initial concentration was obtained in the first phase of the sorption process. During the process, the sorption rate decreased. After about 45 min, the equilibrium was reached. Based on the obtained data, the sorption capacity of raphia fibers in relation to methylene blue increases with increase in the concentration of the dye solution from 100 to 300 mg/dm3. The higher speed of adsorption in the beginning of the process can be connected with the higher amount of free active sites which can be filled with the methylene blue molecules (Fig. 6). The sorption process was carried out with %RSD below 4%.
System with mixing and online measurement
The influence of the sorption process time and the mass of raphia fibers on the sorption capacity of the tested sorbent is shown in Fig. 7. One can see that the dye removal rate is high in the beginning of the adsorption process. State of equilibrium is reached in 80 min for a 1-g bed and 50 min for a 2-g bed. Analyzing the obtained data, it can be summed up that sorption capacity of raphia fibers in relation to methylene blue in the system with mixing and continuous online measurement decreases with increase in the mass of the bed. The increase in the solution concentration will decrease the available sorption surface for the methylene blue. On the other hand, an increase in the mass of sorbent will increase the available surface for the dye. The calculated %RSD for sorption in online system was lower than 5%.
The removal degree of methylene blue (constant concentration of the dye) in relation to the sorbent mass is shown in Fig. 8. It shows that increasing the mass increases the degree of removal, but this is not proportional to the sorbent mass used.
Equilibrium studies
Most of the sorbents used, including natural sorbents, have a porous structure, whereby the rate of removal of impurities from the solution is limited by the diffusion of adsorbate molecules to the interior of the adsorbent pores. In addition, the adsorbate must be transported from the fluid core to the sorbent surface. The adsorption rate depends on the mass transfer coefficient in the solution surrounding the adsorbent and on the adsorbent diffusion coefficient in the porous sorbent. The resistance to external penetration can be reduced by increasing the speed of the solution being purified which is washed by the sorbent. Four models of sorption: Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D-R), were used to evaluate different isotherms to model experimental data (Figs. 9, 10). The aim of the evaluation was to determine the best fit of the isotherm model of the methylene blue sorption.
Table 2 shows summarized values of isotherm model parameters together with the corresponding correlation coefficients R2. The “bath” system of removal of methylene blue by raphia fibers is best characterized by the Langmuir isotherm model showing that the active sites of material used were filled very fast. The pores which are placed inside of the fibers were reached with higher difficulty. The Langmuir isotherm model is the model in which monolayer adsorption onto the surface of homogenous character occurs. The high correlation coefficient for the Freundlich isotherm model may indicate that the Freundlich isotherm is also well adapted. Langmuir isotherm emphasized on the chemisorption and the monolayer adsorption process of sorbate on the surface of adsorbent (Staroń et al. 2017). The model suggests that the used adsorbent have a finite number of active sites, and each site is available for binding only one particle with almost zero interaction between them. In Figs. 9 and 10, the isothermal adsorption curves of dye using four isotherm models at pH 6.5 in 20 °C are shown. As shown in Fig. 9, the experimental data were plotted between Ce and qe for obtaining Langmuir isotherm curve and qmax and KL (the constant related to the energy of adsorption (Dada et al. 2012) parameters for methylene blue were achieved as 34.70 mg/g and 0.0934 L/mg for bath-type adsorption. The isothermal adsorption curves for mixing and the bath system using Freundlich model and the values of 1/n (empirical constant connected to the intensity of adsorption intensity). Freundlich constant is related to the capacity of adsorbent and 1/n parameter is a constant related to the adsorption intensity which varies with the material heterogeneity (Aljeboree et al. 2017) for methylene blue was equal to: 11.5348 mg1−(1/n) (dm3)1/n g−1, 0.2132 mg/g (Bath); 3.3701 mg1−(1/n) (dm3)1/n g−1, 1.7279 mg/g, (Online), respectively. The parameters influencing the sorption process are volume, mass of the sorbent, and the dye concentration. The mass transfer in the case of this study is different, which is reflected in different isothermal models. In the end, it was concluded that the isotherm for methylene blue dye removal by raphia fibers is best fit with the Langmuir isotherm model for bath system. Furthermore, these systems could be sufficiently replaced with the current system using commercial expensive adsorbents.
On the basis of the Langmuir equation, one can calculate the separation coefficient RL; \(R_{\text{L}} = \frac{1}{{1 + K_{\text{F}} C_{0} }}\) (RL = 0—sorption is irreversible, 0 < RL < 1—favorable sorption conditions, RL = 1—linear character of sorption, and RL > 1—unfavorable sorption conditions).
The values of the separation coefficient for the “bath” system for all cases were positive and lower than 1 (0.0917, 0.0621, 0.0455, 0.0365, and 0.0313). This indicates favorable conditions for the methylene blue sorption on the raphia fibers in this system.
Kinetic modeling
The sorption kinetics of basic dye, methylene blue, on raphia fibers are shown in Figs. 11 and 12, respectively. Figure 11 shows the amount of dye sorbed in mg/g raphia against contact time using various concentrations of dye, and Fig. 12 shows the amount of dye sorbed in mg/g raphia against contact time using various mass of sorbent. These plots show that for all solution doses, the amount of dye sorbed increases rapidly with time in the beginning and very slowly toward the end of the sorption process. In the first step, the methylene blue molecules are sorbing onto the surface of raphia where there are no other such molecules, and consequently, the dye–dye interactions are negligible. Visual analysis of the obtained data allows to state that the second-order pseudo-model was the best of the tested models in both studied systems (Tables 3 and 4).
The best fit for all analyzed samples is the pseudo-second-order kinetics model. The results can suggest that sorption process of methylene blue on raffia fibers is of chemical nature. The speed of movement of the solution and the content of methylene blue particles have a significant effect on the mass transfer rate to the adsorbent surface, and therefore, to accelerate the sorption process, it is necessary to ensure intense movement of the liquid that washes the sorbent, also using a high concentration of dye. On the basis of the Weber–Morris model (online), it can be observed that the dye removal process on raphia occurs in two stages. This trend was attributed to the successive saturation of active sites placed on the surface of the organic material available for dye adsorption and to the instauration of a dynamic equilibrium between adsorbed and released molecules (Fabbricino and Pontoni 2016).
Conclusion
The study focuses on the removal of the methylene blue dye through the process of monolayer adsorption on the raphia fibers. Initial concentration, contact time, and bed dose are the main factors on which sorption process is limited.
In the case of the “bath” system, the sorption process occurs quickly, and the sorption equilibrium is established within 45 min in all tested concentrations, and the degree of dye removal ranged from 89 to 49% for concentrations of 100–300 mg/dm3. In the system with mixing and online measurement, the sorption process was carried out longer, the equilibrium stabilized in about 80 min (bed weight 1 g) and about 50 min (bed weight 2 g), and the degree of dye removal ranged from 89 to 92.5% with a bed weight of 1–2 g. Equilibrium studies showed that data obtained for the bath system experiment are best fitted with the Langmuir isotherm, and the system with mixing and online measurement are best fitted with the Freundlich isotherm model. This allows the statement that both systems due to different parameters of sorption processes, including diffusion processes, vary. Studies of sorption kinetics have shown that the pseudo-second-order model best describes the process of sorption of methylene blue on the raphia fibers in both studied systems. On this basis, one can assume that the sorption is of chemical nature.
References
Adeleke JT, Theivasanthi T, Thiruppathi M, Swaminathan M, Akomolafe T, Alabi AB (2018) Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl Surf Sci 455:195–200
Aljeboree AM, Alshirifi AN, Alkaim AF (2017) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:S3381–S3393
Bhatnagar A, Sillanpää M, Witek-Krowiak A (2015) Agricultural waste peels as versatile biomass for water purification—a review. Chem Eng J 270:244–271
Bispo MD, Schneider JK, Silva Oliveira D, Tomasini D, Silva Maciel GP, Schena T et al (2018) Production of activated biochar from coconut fiber for the removal of organic compounds from phenolic. J Environ Chem Eng 6:2743–2750
Chiron N, Guilet R, Deydier E (2003) Adsorption of Cu(II) and Pb(II) onto a grafted silica: isotherms and kinetic models. Water Res 37:3079–3086
Chwastowski J, Staroń P, Kołoczek H, Banach M (2017) Adsorption of hexavalent chromium from aqueous solutions using Canadian peat and coconut fiber. J Mol Liq 248:981–989
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2 + unto phosphoric acid modified rice husk. J Appl Chem 3:38–45
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Elenga RG, Dirras GF, Goma Maniongui J, Djemia P, Biget MP (2009) On the microstructure and physical properties of untreated raffia textilis fiber. Compos Part A Appl Sci Manuf 40:418–422
Fabbricino M, Pontoni L (2016) Use of non-treated shrimp-shells for textile dye removal from wastewater. J Environ Chem Eng 4:4100–4106
Ghosh D, Bhattacharyya KG (2002) Adsorption of methylene blue on kaolinite. Appl Clay Sci 20:295–300
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour Ind 15:14–27
Khanday WA, Marrakchi F, Asif M, Hameed BH (2017) Mesoporous zeolite—activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J Taiwan Inst Chem E 70:32–41
Kumpan N, Poonsawat T, Chaicharoenwimolkul L, Pornsuwana S, Somsook E (2017) Ferrocenated nanocatalysts derived from the decomposition of ferrocenium in basic solution and their aerobic activities for the rapid decolorization of methylene blue and the facile oxidation of phenylboronic acid. RSC Adv 7:5759–5763
Mouni L, Belkhiri L, Bollinger JC, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of Methylene Blue from aqueous solutions by adsorption n Kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Nataraj SK, Hosamani KM, Aminabhavi TM (2009) Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures. Desalination 249:12–17
Nigam P, Armour G, Banat IM, Singh D, Marchant R (2000) Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour Technol 72:219–226
Pal K, Ghorai K, Aggrawal S, Mandal TK, Mohanty P, Seikh MM, Gayen A (2018) Remarkable Ti-promotion in vanadium doped anatase titania for methylene blue adsorption in aqueous medium. J Environ Chem Eng 6:5212–5220
Qiu H, Lv L, Pan BC, Zhang QI, Zhang WM, Zhang QX (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10:716–724
Rahmi Ishmaturrahmi, Mustafa I (2019) Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem J 144:397–402
Ramesh A, Hasegawa H, Maki T, Ueda K (2007) Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Sep Purif Technol 56:90–100
Rane NR, Chandanshive VV, Khandare RV, Gholave AR, Yadav SR, Govindwar SP (2014) Green remediation of textile dyes containing wastewater by Ipomoea hederifolia L. RSC Adv 69:36623–36632
Safavi A, Momeni S (2012) Highly efficient degradation of azo dyes by palladium/hydroxyapatite/Fe3O4 nanocatalyst. J Hazard Mater 201–202:125–131
Staroń P, Chwastowski J, Banach M (2017) Sorption and desorption studies on silver ions from aqueous solution by coconut fiber. J Clean Prod 149:290–301
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562
Yang Y, Lin X, Wei B, Zhao Y, Wang J (2014) Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int J Environ Sci Technol 11:1093–1100
Acknowledgements
This work is a part of the project Removal of organic compounds from wastewater by sorption process using plant material supported by Ministry of Science and Higher Education under the Project C-1/288/2016/DS-M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Staroń, P., Chwastowski, J. & Banach, M. Sorption behavior of methylene blue from aqueous solution by raphia fibers. Int. J. Environ. Sci. Technol. 16, 8449–8460 (2019). https://doi.org/10.1007/s13762-019-02446-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02446-9