Abstract
Bio-removal of heavy metals, using microbial biomass, increasingly attracting scientific attention due to their significant role in purification of different types of wastewaters making it reusable. Heavy metals were reported to have a significant hazardous effect on human health, and while the conventional methods of removal were found to be insufficient; microbial biosorption was found to be the most suitable alternative. In this work, an immobilized microbial consortium was generated using Statistical Design of Experiment (DOE) as a robust method to screen the efficiency of the microbial isolates in heavy metal removal process. This is the first report of applying Statistical DOE to screen the efficacy of microbial isolates to remove heavy metals instead of screening normal variables. A mixture of bacterial biomass and fungal spores was used both in batch and continuous modes to remove Chromium and Iron ions from industrial effluents. Bakery yeast was applied as a positive control, and all the obtained biosorbent isolates showed more significant efficiency in heavy metal removal. In batch mode, the immobilized biomass was enclosed in a hanged tea bag-like cellulose membrane to facilitate the separation of the biosorbent from the treated solutions, which is one of the main challenges in applying microbial biosorption at large scale. The continuous flow removal was performed using fixed bed mini-bioreactor, and the process was optimized in terms of pH (6) and flow rates (1 ml/min) using Response Surface Methodology. The most potential biosorbent microbes were identified and characterized. The generated microbial consortia and process succeeded in the total removal of Chromium ions and more than half of Iron ions both from standard solutions and industrial effluents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Importance and pollution of River Nile
Due to its vital values for life, water is required in a cleaner form where plants, animals and humans cannot survive if it is loaded with high metal concentration, pathogenic microorganisms and/or hazard chemicals (Frick et al. 1999).
The River Nile is the crucial life artery of Egypt, as it is the main source of freshwater for irrigation, industry, domestic uses, a source of power from the hydroelectric generation facility at Aswan, and a mean of transportation for goods and people (Afifi et al. 2016). The River Nile enters Egypt at Adindan village of Nubia (where lake Nasser started) and runs northward till Cairo for about 1188 km. From Cairo, it streams northwestward for a distance of about 23 km where Nile delta begins and the river splits into Damietta (eastern) branch (about 242 km) and Rosetta (western) branch (about 236 km) (Cumberlidge 2009; Rzóska 2012). Egypt was considered among the ten countries to be suffered from freshwater shortage by the year 2025 due to the rapidly increasing population and limited resources of water (Engelman and LeRoy 1993).
By the beginning of fifties of the 20th century, heavy industrialization was introduced to Egypt along the Nile, in Delta, Cairo and Alexandria. Food, Metal Products, Chemicals and Textile Industries are the most dominant industrial activities in Egypt (Saeed and Shaker 2008). In Egypt, it was reported that approximately 350 industrial facilities that are discharging their wastes either directly or indirectly into the Nile or through the municipal system (Abdel-Shafy and Aly 2002).
Unquestionably, the influences of industrial pollution in Egypt affect all environments including water, air and/or land. Industrial pollutants contaminating the surface water caused deadly effects on structure and function of resident biological communities (Abdel-Satar 2005; Faragallah et al. 2009). These facts about the present situation of water quality of the River Nile indicated that obtaining a clean and safe drinking water may represent a potential challenge at places suffering from excessive loads of pollutants.
Industrial wastewater treatment is one of the hottest topics within the scientific community, and a considerable effort was generated for such processes especially for the issue of heavy metal contamination. A high concentration of heavy metals in water supplies is undesirable because of their potential adverse effects on health, environment and corrosion of pipelines. Heavy metals are very toxic to human beings. Muscular and cardiovascular disorders, liver, brain and kidney damages are all triggered by heavy metals in drinking water (Sawyer and McCarty 1978).
Heavy metal removal
Processes for eliminating metal ions from aqueous solution generally consist of physical, chemical and biological technologies. Standard methods for removing metal ions from aqueous solution involve chemical and electro coagulation, filtration, membrane technologies, chemical precipitation, electrochemical treatment, ion exchange, adsorption on activated carbon, zeolite and evaporation, etc. (Ali and Gupta 2006). Recently, some wastes were found to have a significant efficacy in removing different types of heavy metals including Lead (II) and Nickel (II) (Olgun and Atar 2012). However, electrochemical treatment and chemical precipitation are ineffective, and also produce huge quantity of sludge which is required to be treated with great difficulty. Activated carbon adsorption process, ion exchange and membrane technologies are extremely expensive when treating large amount of wastewater and water containing heavy metal in low concentration as a result cannot be employed at large scale (Fu and Wang 2011).
The development and implementation of cost-effective, new processes and the application of new generation adsorbents for removal/recovery of metals are critical to upgrade the competitiveness of industrial processing operations (Ali 2012). Disadvantages, together with the need for more economical and effective methods for the recovery of metals from wastewaters, resulted in the development of alternative separation technologies (Wang and Chen 2006; Ali 2010).
Bisorption
Biosorption, a passive and metabolic independent process, could be characterized as the removal of heavy metals using a passive binding process with non-living microorganisms including algae, bacteria, filamentous fungi, and yeasts (Parvathi and Nagendran 2007), and other biomass types that are capable of efficiently collecting heavy metals.
The biosorption process showed many desirable features including its rapid kinetics of adsorption and desorption, the selective removal of metals over a wide range of pH and temperatures in addition to the low capital and operation cost. Biosorbent could easily be produced using low-cost growth media or obtained as an industrial by-product (Ahluwalia and Goyal 2007; Ali et al. 2012). Based upon the metal binding capacities of various biological materials, biosorption could separate heavy metals from various waste materials including wastewater effluent even at low concentrations (Vilar et al. 2007).
The main advantages of biosorption over conventional treatment methods includes: high removal efficiency for low concentration solutions, low cost, no additional nutrients required, a minimal amount of chemical and/or biological sludge and the possibility of biosorbent regeneration and metal recovery (Vilar et al. 2007). The sorption of heavy metals onto these biomaterials is attributed to their constituents, which are mainly carbohydrates, proteins and phenolic compounds, since they contain functional groups such as hydroxyls, carboxyls and amines which were proven to have a vast capability to attach to the metal ions (Choi and Yun 2006).
Biosorption process could be affected by numerous factors which usually related to either the biomass and the metal or the surrounding environmental conditions, and these major factors are: pH, temperature, nature of the biomass, biomass concentration, initial concentration and metal affinity to the biosorbent (Ahalya et al. 2003; Malkoc and Nuhoglu 2005). Different species of bacteria, yeast and filamentous fungi were proven to be a potential biosorbent and were reported to remarkably remove heavy metals from different waste effluents (Malkoc and Nuhoglu 2005).
Heavy metal removal was reported to be significantly enhanced upon optimization of all the variables affecting the removal processes and could brought down to the ppb level (Ali et al. 2011).
The main goal of this work is to develop a robust method to generate a microbial mixture (bacteria and fungi) that could efficiency remove heavy metals from industrial effluents by applying statistical design of experiment as a biosorption screening tool and to test the ability of the generated microbial mixture in removing metal ions from real industrial effluents collected from Egyptian water main stream. Acheiving these targets may participate in solving the deadly waste problem of the river Nile which threaten the life of the Egyptian people.
Materials and methods
Samples collection and isolation of the heavy metal resistant microbes
Water samples were collected from the industrial effluents discharge pipe of four different factories in Al Dakhliya governorate, Egypt; these are: Delta Fertilizer Factory (DFF), Talkha Electric Power Plant (TEP), Marble and Granite Factory (MGF) and Sandoup Oil and Soup Factory (SOSF). In addition to water, the sediments near to the discharge pipes were collected and prepared as reported by Webster (2008).
Heavy metal contents of the wastewater were determined using atomic absorption spectro-photometer type Buck scientific accusys 214/215 according to Wirsen and Jannasch (1976).
To isolate resistant microbes from the collected samples, one ml of each of the collected samples was inoculated to a nutrient agar medium (to isolate resistant bacteria) and Potato Dextrose agar (PDA) medium (to isolate resistant fungi). These media were amended with 100 ppm of Cr (VI) and 100 ppm of Pb (II) separately. The inoculated bacterial plates were incubated at room temperature (30–35 °C) for 48 h. The bacterial colonies were collected, subcultured and purified, and each colony was preserved in 20% glycerol at −80 °C for further work. For fungal isolation, the plates were incubated of at 28 °C for 7 days and the fungal colonies were collected, sub-cultured, purified and were preserved on PDA slants for further work.
Screening for the most potential biosorbent microbes
Eighteen different microbes were screened for their capability to work as a possible biosorbent for Chromium (VI) and Lead (II) removal from industrial effluent samples. Seventeen of them were isolated from the collected wastewater samples and were found to have a relatively high resistant against high concentrations of the tested metals during the Minimal Inhibitory Concentration (MIC) experiments (data not shown). In addition to the isolated resistant microbes, a commercially bakery yeast powder was used as a positive control microbe due to its high capability to remove heavy metals (Wang and Chen 2006).
The tested bacterial isolates were grown in broth media (50 ml in each flasks) at room temperature in orbital shaking incubator till the late log phase (constant OD600 = 1), and then the bacterial biomass of each was immobilized using alginate beads as described by Yakup Arıca et al. (2004). Regarding the resistant fungi, the tested fungal isolates were grown on solid media and a spore suspension (the concentrations of the spore suspensions were determined in a haemocytometer and adjusted to 1.0 to 2.5 × 106) for each was prepared and immobilized using alginate beads. The beads containing the proposed biomass were pretreated with 0.1 M HCl as described by Aqeel Ashraf et al. (2012).
Afterward, the pretreated beads containing the proposed microbial biomass and fungal spore suspensions were packaged in Cellulose tissue that was wrapped into a “tea-bag”-like configuration (4 × 4 cm). The tea bag containing the beads was fixed into the middle of 250 ml glass beaker flask containing standard heavy metal solution as described in Fig. 1 with continuous stirring.
The screening strategy was performed using a level III resolution Plackett–Burman design of experiment which was found to be efficient in various screening processes (Plackett and Burman 1946). Plackett–Burman design of experiment depends on testing the effect of the variables on two levels (minimum and maximum levels); the maximum value of the tested microbes was estimated to be 1 g of the immobilized biomass in the performed trials, while the minimum value of the tested microbe was calculated as 0.1 g of the immobilized biomass in the performed trials. Table 1 represents the generated matrix and shows the performed trials to screen the capability of the tested isolates in the proposed heavy metal removal. A dummy variable (empty alginate beads) was used to evaluate the standard error of the design and as a blank to confirm that the biosorption capability was related to the biomass within the beads.
The screening experiments were performed with a prepared heavy metal solution with 100 ppm as an initial concentration for each of the targeted heavy metals (CrIV and PbII) separately. The immobilized biomass mixtures (as described in Table 1) were immersed in the heavy metal solution (batch mode as shown if Fig. 1) with continues stirring, and the remaining heavy metal concentrations were measured (via atomic absorption method), and the percentages of heavy metal removal were calculated as described in the following equation:
where C (ppm) is the initial metal ion concentration, Ci (ppm) is the metal ion concentration after biosorption. All the biosorption experiments were repeated three times to confirm the results. Also, blank experiments were conducted to ensure that no adsorption had taken place on the walls of the apparatus used.
Identification of the most potential isolates
The most significant microbes (bacteria and fungi) that were reported to be able to remove heavy metal from the standard aqueous solutions were identified as following.
Bacterial identification
The Insta-Gene Matrix Genomic kit (Bio-Rad, USA) was used to extract the total genomic DNA from the isolates under investigation and used as a template for amplification of the 16S rRNA gene. PCR reaction was prepared as followed: 5 µl master mix, 20 µl each of primers 518F (5′- CCA gCAgCCgCggTA ATA Cg -3′) and 800R (5′-TAC CAgggT ATC TAA TCC -3′), 3 µl of 50 mM MgCl2, 0.5 µl of AmpliTaq DNA polymerase, and 1 µl of genomic DNA, and the total volume was made up with distilled water to 50 µl. The PCR products were checked by agarose gel electrophoresis (1% w/v; 30 min at 100 V, 0.59 TBE).The amplified fragments were compared with 100 bp molecular size marker (MBI Fermentas, Lithuania). The PCR product was stored at −20 °C.
The PCR product was purified using Montage PCR Clean up kit (Millipore) before being sequenced. Sequencing was performed by using ABI PRISM®BigDyeTM Terminator Cycle Sequencing Kits. A 2 µl of the kit was mixed with 5 µl of PCR mixture in a 1.5 ml sterile screw tube, incubated for 15 min at 37 °C, followed by a second incubation for 15 min at 80 °C. The purified PCR product was then sequenced using an Applied Biosystem model 3730XL automated DNA sequencing system (Applied BioSystems, USA). The quality and quantity of the sequence obtained were checked with Finch TV version 1.4.0. While, DNA baser (version 3.55.0.199) software was used to assemble the gene. The sequence was identified by BLAST and SeqMatch against Genbank database.
Fungal identification
Potato Dextrose Agar (PDA) was used for culturing fungi. The most potential fungi were identified based on the observation of cultural and morphological characteristics, color of colony and sporulation. Examination was carried out using needle-mount preparation whereby fragments of the sporing surface of the culture was taken. This was teased out in drop of alcohol on a cleaned glass slide using needle. The fragment was stained by adding a drop of lactophenol. A cover slip was applied carefully avoiding air bubbles, and the preparation was examined under light microscope (Barnett and Hunter 1972).
Time trajectory for heavy metal removal in batch mode
Using the same setting (as shown in Fig. 1), the most potential microorganisms immobilized biomasses were used as microbial consortia to remove the targeted heavy metals separately. For Pb (II) removal, a microbial consortia of (Pb26BSP, Pb3BA, Cr56FSU and Saccharomyces), while for Cr (IV) removal, a microbial consortia of (Cr3BA, Cr5FA, Pb26BSP, Cr15BW, Pb11BW and Pb55BSU) were used. The initial heavy metal concentration was 100 ppm, and samples were taken at time intervals of (0, 5, 10, 15, 25, 35, 40 and 45 min), and subsequently, the concentration of each heavy metal was measured using the atomic absorption set and rate of heavy metal removal was calculated.
After testing the capability of the generated microbial consortia to remove the targeted heavy metal ions from the prepared solution, the same setting was performed using the industrial waste effluents to confirm the capability to remove the targeted heavy metals from the actual wastes.
Continuous flow removal of heavy metals and its optimization
A continuous removal of the targeted heavy metals, from selected industrial effluents, was performed using a fixed bed mini-reactor with chosen immobilized biomass of the most potential isolates; the setup of the continuous removal is shown in Fig. 2. Different initial pH and flow rates were used by generating a Central Composite Design matrix (Table 3) to determine the optimum removal conditions when applying such reactor in heavy metal removal processes.
In order to optimize the heavy metal removal, two variables (flow rate and pH) were optimized using a Central Composite Design (CCD) with five levels for each variable. The tested pH ranges from 5.5 to 8.5, and the tested flow rates ranges from 1 to 15 ml/min.
Results and discussion
Wastewater sample collection and isolation of the heavy metal resistant microbes
From the collected effluents and sediments, One hundred and two bacterial strains and one hundred and eleven fungal strains were isolated on nutrient media containing 100 ppm of Cr (IV) and Pb (II) separately to ensure the preselection of potential heavy metal resistant isolates.
Screening for the most potential biosorbent microbes
Plackett–Burman design of experiments was applied to screen the most potential microbial isolates for their biosorption capability. Plackett–Burman design of experiments is a level III design which determines the main effect of a large number of variables on a certain process and was reported to be efficient in a vast number of microbial processes (El razak et al. 2014). The microbial mixtures were prepared (according to the generated matrix in Table 1) and applied in a batch mode to examine the efficiency of the tested microbial mixtures to remove Cr (IV) and Pb (II) from the prepared solution. The rate of removal, of each of the targeted ions, in each trial was calculated and used as a response (Table 1) to be analyzed and maximizing the removal rate capability as a target.
Eighteen different isolated microbes were used to perform such experiment. They were selected due to their capability to grow and resist relatively high concentrations of the targeted heavy metals when amended to the cultivation media. The used biomass was pretreated bacterial biomass and fungal spores.
In addition to the isolated heavy metal resistant microbes, dried immobilized bakery yeast biomass was used as a positive control, as yeast was previously reported to remove a vast number of heavy metals from aqueous solutions in an efficient way (Ahluwalia and Goyal 2007; Wang and Chen 2006). A dummy variable (empty alginate beads) was used to evaluate the standard error of the design and as a blank to confirm that the biosorption capability was related to the biomass within the beads. The tested isolates were selected according to their relative high resistant against high concentrations of the tested metals during the preliminary Minimal Inhibitory Concentration (MIC) experiments (data not shown).
The Chromium ions were totally removed in most of the trials indicating the successful ability of the generated microbial mixture to remove such heavy metal from aqueous solutions. Although the microbial mixtures succeed in removing the Chromium ions completely, the removal rate of Lead ions did not exceed 75% (Trial number 2, at Table 1) which considered as a satisfied rate.
One of the main challenges in applying biomass for heavy metal removal in industrial scale is recollection of the used biomass after the heavy metal removal process. In this work, the immobilized biomass was used for not contaminating the outlet effluent and these immobilized biomasses were hanged in a tea bag-like tissue (as shown in Fig. 1) to facilitate the collection process and separate them from the treated solution (industrial waste).
The used biomass (bacterial biomass and fungal spores) were pretreated by soaking in a light acidic solution which lead to enhance the biosorption capability and remove any by-products which may have a hazardous effect on the environment (Aqeel Ashraf et al. 2012).
The obtained data were exposed to a multi-way ANOVA analysis to explore the most significant microbes with a potential capability to remove the tested heavy metals (Table 2).
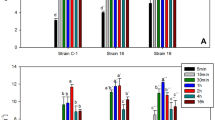
According to the statistical analysis, microbial biomass with significant effect on heavy metal removal had a P value <0.05 (marked with bold and asterisk) for a 95% level of confidence. Pareto-chart (Fig. 3) summarize the biomass with significant effect on heavy metal removal (significant variables are crossing the red line), where (a) showed the organisms that significantly remove Cr (IV) ions while (b) showed the organisms that significantly remove Pb (II) ions from the prepared aqueous solution.
According to the results obtained, the following organisms including; Cr3BA, Cr5FA, Pb26BSP, Cr15BW, Pb11BW and Pb55BSU were found to be the most potential microbes to be efficient in removing Chromium ions, while organisms Pb26BSP, Pb3BA, bakery yeast and Cr56FSU were found to be the most potential microbes to be efficient in removing Lead ions. All of the selected organisms showed a statistically significant effect on removing the targeted heavy metal ions with P values <0.05 at level of confidence of 95% except the isolate Cr56FSU which had a P value of 0.054, which was very close to be significant, so it was decided for not neglecting its efficacy especially that for Pb (II) ions removal only three isolates were found to be significant.
According to our results, the selected potentials microbes for the removal of Cr (IV) ions were found to be even better than bakery yeast (the positive control) although its known efficacy in heavy metal removal. For Pb (II) ions removal, bakery yeast came as third after the isolates Pb26BSP and Pb3BA which were reported to be more significantly efficient in heavy metal removal capability.
Time trajectory for heavy metal removal in batch mode
The selected potential microbes were used as consortia to remove each of the tested metal ions separately. A batch mode was applied (as shown in Fig. 1), using the same settings, to remove the tested heavy metals from industrial effluents. The heavy metal ions concentrations were monitored and tested at time intervals. Figure 4 shows the heavy metal removal over time using the proposed microbial mixtures.
The used microbial consortium achieved a 100% removal of Cr (IV) ions from the industrial effluents within 10 min (approximately 60% after 5 min), while only 55% of Pb (II) ions were removed from the industrial effluents in the proposed process.
Applying the immobilized microbial mixture, hanged in the tea bag like, on removing heavy metal from industrial effluent showed a complete removal of Cr (IV) ions and removed half of the Pb (II) ions which indicate the efficiency of the generated consortia in removing the targeted metals even in the presence of a mixture of heavy metals (Iron, Chromium, Cadmium, Nickel, Copper, Cobalt and zinc). The capability of the generated consortia to remove the target ions indicates its high selectivity which could be considered as a significant criteria.
Continuous flow removal of heavy metals and its optimization
The continuous removal of the target heavy metals was performed as with the setup as shown in Fig. 2. Continuous removal using adsorption column was proven to be the most efficient method in heavy metal removal at different scales including pilot and industrial scales (Ali 2014). Fixed bed reactors were proven to be an efficient tool to remove many wastes and ions including Lead, Nickel, Phosphate and Nitrate (Olgun and Atar 2012; Olgun et al. 2013) The generated microbial consortium was loaded in fixed bed reactor, and the industrial effluents passed through the column then the amount of the adsorbed metals were calculated.
In order to enhance the heavy metal removal during the continuous process, different variables including different flow rates and initial pH (as shown in Table 3) were examined to determine the optimum condition for heavy metal removal by the proposed microbial biomass consortia and maximize their biosorption capability.
Even in continuous removal, Cr (IV) ions were nearly completely removed (98% removal in Trial number 7) from the prepared solution with pH = 6 and flow rate of 5 ml/min as the best tested conditions. The same conditions achieved 55% removal of Pb (II) ions from the prepared solution. Central composite design was reported to determine the precise optimum conditions for different microbial processes on different scales (Abd Elrazak et al. 2013).
The obtained responses were exposed to multi-way ANOVA analysis (Table 4) to determine the significance effect of each of the tested variables, their interaction and their quadratic effect. According to the statistical analysis, the Model F value of 35.37 implies the model is significant. There is only a 0.01% chance that a “Model F value” this large could occur due to noise. Values of “Prob > F” <0.05 indicate model terms are significant.
Regarding Pb (II) ion removal, the main effect of pH was found to be insignificant while its quadratic effect was reported to be significant. While, the main effect of pH was reported to be statistically significant in the process of Cr (IV) ion removal, its quadratic effect was reported to be insignificant. Both the main effect and the quadratic effect of the flow rates were found to be statistically significant in the process of Cr (IV) and Pb (II) ions removal. Unexpectedly, the interaction between the tested variables was found to be statistically insignificant at the used level of confidence.
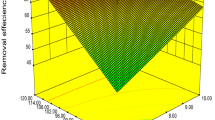
The relation between the tested variables and each of the calculated responses could be clarified in Fig. 5.
Using the generated mathematical model and graphs, the optimum removal conditions were found to be pH = 6–7 and flow rate = 1–5 ml/min. Slow flow rate is expected to be preferred to achieve better removal in order to give enough contact time between the effluent and the biomass. The optimum pH was approximately neutral (>5) which could be due to increasing the density of the negative charge on the surface of the biosorbent giving way to more heavy metal ions being adsorbed from the solution (Atar et al. 2012). In the meantime, the obtained result contradict with the result obtained by (Ilhan et al. 2004) as they found that the optimum pH for Cr (IV) and Pb (II) ions removal were 2 and 4.5, respectively, when using Staphylococcus saprophyticus as a biosorbent and applying one factor at a time as an optimization methodology. The better efficiency of heavy metal removal at pH > 4 could be due to the activation of the functional groups on the active sites of the biosorbent.
Identification of the most potential biosorbent microbes
The bacterial isolates used in the biosorption process were identified via sequencing the 16S rRNA gene and its analysis as described in “Bacterial Identification” section and aligned with the closest type strains as shown in Fig. 6.
The isolates Pb26BSP and Pb3BA were found to be closely related to the genus Vibrio. Isolate Pb55BSU was more related to the genus Serratia, isolate Pb11BW and Cr3BA were more closely related to genus Bacillus, while isolate Cr15BW was closest to the genus Paenabacillus.
According to the method described at “Fungal identification” section, the two selected fugal isolates Cr5FA and Cr56FSU were identified to be Trichoderma viride and Fusarium oxysporium, respectively.
Conclusion
Heavy metals were always reported to be a serious problem due its hazardous health effect on human body. For countries like Egypt, which is expected to face a serious water lack problem in the near future, wastewater recycling became a must. Biosorption was reported to be a potential strategy to remove toxic heavy metals or even reduce the concentration of such elements into its minimum accepted values. Microbial biomass was reported to successfully remove various types of heavy metals both in batch and continuous processes (Çolak et al. 2013).
In this research, a robust methodology was developed, for the first time, to screen the efficiency of a large number of microbial strains, isolated from the industrial effluents and sediment, to remove Lead and Chromium ions from the industrial effluents of four different factories. Plackett–burman, which was proven to be a significant methodology to screen the effect of different variables including culture conditions and medium components, was used in this work to screen the efficacy of microbes in removing heavy metals from industrial effluents for the first time in the literature.
The empty beads were used as a Dummy variable (negative control) to ensure that the significant of the effect of the biosorbent microbes is not related to the beads biosorption capability. Bakery yeast, a known potential biosorbent, was used as a positive control to compare the ability of the selected isolates to remove the targeted heavy metals with.
Immobilizing the immobilized biomass in a tea bag-like membranes is expected solve one of the main obstacles in applying microbial biomass at large scale, where the removal of the used biosorbent and its separation from the treated solution will be more efficient and simpler.
Six different bacterial and two fungal isolates were found to be a potential consortium to efficiently remove heavy metals from industrial effluents, as this generated consortium succeeded in removing all the Chromium ions and more than half of the Iron ions from both the prepared standard solutions and the real industrial effluents even in the presence of other heavy metals. The generated consortium achieved the desired target when introduced both in batch and/or continuous modes.
For the future work, the mechanism of action of this consortium will be studied and explained which could be challenging.
References
Abd Elrazak A, Ward A, Glassey J (2013) Response surface methodology for optimising the culture conditions for eicosapentaenoic acid production by marine bacteria. J Ind Microbiol Biotechnol 40:477–487. doi:10.1007/s10295-013-1238-x
Abdel-Satar AM (2005) Quality of river Nile sediments from Idfo to Cairo. Egypt J Aquat Res 31:182–199
Abdel-Shafy H, Aly R (2002) Water issue in Egypt: resources, pollution and protection endeavors. Navigation 49:4–6
Afifi R, Nagwa E, Amro H, Martin H, Bo M, Helmy O, Alharbi O, Ali I (2016) SPE and HPLC monitoring of 17-β-estradiol in Egyptian aquatic ecosysetms. J Liq Chromatogr Relat Technol 8:428–434. doi:10.1080/10826076.2016.1174712
Ahalya N, Ramachandra T, Kanamadi R (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257. doi:10.1016/j.biortech.2005.12.006
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purif Rev 39:95–171. doi:10.1080/15422119.2010.527802
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091. doi:10.1021/cr300133d
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43(3):175–205. doi:10.1080/15422119.2012.748671
Ali I, Gupta V (2006) Advances in water treatment by adsorption technology. Nat Protoc 6:2661–2667. doi:10.1038/nprot.2006.370
Ali I, Khan T, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep Purif Rev 40(1):25–42. doi:10.1080/15422119.2011.542738
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183. doi:10.1016/j.jenvman.2012.08.028
Aqeel Ashraf M, Maah J, Yusoff I (2012) Removal of lead from synthetic solutions by protonated teleosts biomass. J Chem 9:345–353. doi:10.1155/2012/769180
Atar N, Olgun A, Wang S (2012) Adsorption of cadmium(II) and zinc(II) on boron enrichment process waste in aqueous solutions: batch and fixed-bed system studies. Chem Eng J 192:1–7. doi:10.1016/j.cej.2012.03.067
Barnett HL, Hunter BB (1972) Illustrated genera of imperfect fungi illustrated genera of imperfect fungi. 930–932
Choi SB, Yun YS (2006) Biosorption of cadmium by various types of dried sludge: an equilibrium study and investigation of mechanisms. J Hazard Mater 138:378–383. doi:10.1016/j.jhazmat.2006.05.059
Çolak F, Olgun A, Atar N, Yazıcıoğlu D (2013) Heavy metal resistances and biosorptive behaviors of Paenibacillus polymyxa: batch and column studies. J Ind Eng Chem 19(3):863–869. doi:10.1016/j.jiec.2012.11.001
Cumberlidge N (2009) Freshwater crabs and shrimps (Crustacea: Decapoda) of the Nile basin. In: The Nile, Springer, Netherlands pp 547–561. doi:10.1007/978-1-4020-9726-3_27
El razak AA, Ward AC, Glassey J (2014) Process development of eicosapentaenoic acid production. Biochem Eng J 82:53–62. doi:10.1016/j.bej.2013.10.022
Engelman R, LeRoy P (1993) Sustaining water. Population and the future of renewable water supplies. Popul Dev Rev 19:1–32
Faragallah H, Askar A, Okbah M, Moustafa H (2009) Physico-chemical characteristics of the open Mediterranean sea water far about 60 Km from Damietta harbor. Egypt J Ecol Nat Environ 1:106–119
Frick C, Germida J, Farrell R (1999) Assessment of phytoremediation as an in situ technique for cleaning oil-contaminated sites. Proceedings of the phytoremediation Technical seminar. Environment Canada, pp 105–124
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418. doi:10.1016/j.jenvman.2010.11.011
Ilhan S, Nourbakhsh MN, Kiliçarslan S, Ozdag H (2004) Removal of chromium, lead and copper ions from industrial waste waters by Staphylococcus saprophyticus. Turk Electron J Biotechnol 2:50–57
Malkoc E, Nuhoglu Y (2005) Investigations of nickel(II) removal from aqueous solutions using tea factory waste. J Hazard Mater 127:120–128. doi:10.1016/j.jhazmat.2005.06.030
Olgun A, Atar N (2012) Equilibrium, thermodynamic and kinetic studies for the adsorption of lead(II) and nickel(II) onto clay mixture containing boron impurity. J Ind Eng Chem 18(5):1751–1757. doi:10.1016/j.jiec.2012.03.020
Olgun A, Atar N, Wang S (2013) Batch and column studies of phosphate and nitrate adsorption on waste solids containing boron impurity. Chem Eng J 222:108–119. doi:10.1016/j.cej.2013.02.029
Parvathi K, Nagendran R (2007) Biosorption of chromium from effluent generated in chrome-electroplating unit using Saccharomyces cerevisiae. Sep Sci Technol 42:625–638. doi:10.1080/01496390601070158
Plackett RL, Burman JP (1946) The design of optimum multifactorial. Exp Biom 33:305–325. doi:10.1093/biomet/33.4.305
Rzóska J (2012) The Nile, biology of an ancient river: biology of an ancient river, vol 29. Springer Science & Business Media, Berlin
Saeed SM, Shaker IM (2008) Assessment of heavy metals pollution in water and sediments and their effect on Oreochromis niloticus in the northern delta lakes, Egypt. In: 8th International Symposium on Tilapia in Aquaculture, pp 475–490
Sawyer CN, McCarty PL (1978) Chemistry for environmental engineers. Mc Graw-Hill Book Company, New York
Vilar VJ, Botelho CM, Boaventura RA (2007) Modeling equilibrium and kinetics of metal uptake by algal biomass in continuous stirred and packed bed adsorbers. Adsorption 13:587–601. doi:10.1007/s10450-007-9029-1
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. doi:10.1016/j.biotechadv.2006.03.001
Webster R (2008) Soil sampling and methods of analysis: edited by MR Carter and EG Gregorich. Eur J Soil Sci 59:1010–1011. doi:10.1111/j.1365-2389.2008.01052_5.x
Wirsen CO, Jannasch HW (1976) Decomposition of solid organic materials in the deep sea. Environ Sci Technol 10:880–886. doi:10.1021/es60120a002
Yakup Arıca M, Bayramoǧlu G, Yılmaz M, Bektaş S, Genç Ö (2004) Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J Hazard Mater 109:191–199. doi:10.1016/j.jhazmat.2004.03.017
Acknowledgements
Authors want to acknowledge the managers of the factories included in the study for their help in collecting the samples from the discharge pipes and facilitate all the paper work required for such process.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Xu Han.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Migahed, F., Abdelrazak, A. & Fawzy, G. Batch and continuous removal of heavy metals from industrial effluents using microbial consortia. Int. J. Environ. Sci. Technol. 14, 1169–1180 (2017). https://doi.org/10.1007/s13762-016-1229-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1229-3