Abstract
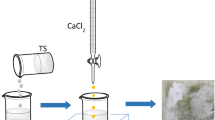
This paper investigates the potential of alginate-immobilised Chlorella sorokiniana for removing Cu2+, Ni2+ and Cd2+ ions from drinking water solutions. The effects of initial metal concentrations, contact times and temperatures on the biosorptions and removal efficiencies of the tested metals were investigated at initial pH values of 5, and pH effects were studied within the range of 3–7. When studying the effects of initial metal concentrations, the highest experimental removal yields achieved for Cu2+, Ni2+ and Cd2+ ions were 97.10, 50.94 and 64.61 %, respectively. The maximum biosorption capacities obtained by the Langmuir isotherm model for the biosorptions of Cu2+, Ni2+ and Cd2+ ions by alginate-immobilised C. sorokiniana were found to be 179.90, 86.49 and 164.50 mg/g biosorbent, respectively. The experimental data followed pseudo-second-order kinetics. At an initial metal concentration of 25 mg/L, immobilised algae could be used in at least 5 successive biosorption–desorption cycles. SEM and EDS analyses revealed that the metals bonded to the biosorbent. Bi- and multi-metal systems of Cu2+, Ni2+ and Cd2+ were investigated at initial metal concentrations of 30, 50 and 100 mg/L. The removal of Cd2+ as well as Ni2+ in such systems was negatively affected by the presence of Cu2+. The removal efficiency for Cu2+ in multi-metal systems decreased by 5–7 %, whilst in the cases of Cd2+ and Ni2+ the efficiencies decreased by up to 30 %. Nevertheless, the results obtained show that alginate-immobilised C. sorokiniana can efficiently remove the metals tested from polluted drinking water sources.






Similar content being viewed by others
References
Abdel-Ghani NT, El-Chaghaby GA (2014) Biosorption for metal ions removal from aqueous solutions: a review of recent studies. Int J Latest Res Sci Technol 3:24–42
Akhtar N, Saeed A, Iqbal M (2003) Chlorella sorokiniana immobilized on the biomatrix of vegetable sponge of Luffa cylindrica: a new system to remove cadmium from contaminated aqueous medium. Bioresour Technol 88:163–165. doi:10.1016/S0960-8524(02)00289-4
Akhtar N, Iqbal J, Iqbal M (2004) Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: characterization studies. J Hazard Mater 108:85–94. doi:10.1016/j.jhazmat.2004.01.002
Akhtar N, Iqbal M, Zafar SI, Iqbal J (2008) Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). J Environ Sci 20:231–239. doi:10.1016/S1001-0742(08)60036-4
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294. doi:10.1016/S1383-5866(00)00212-4
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem 38:89–99. doi:10.1016/S0032-9592(02)00051-1
Aksu Z, Dönmez G (2006) Binary biosorption of cadmium(II) and nickel(II) onto dried Chlorella vulgaris: co-ion effect on mono-component isotherm parameters. Process Biochem 41:860–868. doi:10.1016/j.procbio.2005.10.025
Al-Rub FAA, El-Naas MH, Benyahia F, Ashour I (2004) Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem 39:1767–1773. doi:10.1016/j.procbio.2003.08.002
Al-Rub FAA, El-Naas MH, Ashour I, Al-Marzouqi M (2006) Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem 41:457–464. doi:10.1016/j.procbio.2005.07.018
Bayramoğlu G, Yakup Arıca M (2009) Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): kinetics and equilibrium studies. Bioresour Technol 100:186–193. doi:10.1016/j.biortech.2008.05.050
Bischoff H, Bold HC (2003) Psychological studies IV. Some soil algae from enchanted rock and related algal species, vol 6318. University Texas Publications, Austin
Blanes P et al (2011) Biosorption of trivalent chromium from aqueous solution by red seaweed Polysiphonia nigrescens. J Water Resource Prot 3:832–843
Bulgariu D, Bulgariu L (2012) Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour Technol 103:489–493. doi:10.1016/j.biortech.2011.10.016
Carfagna S, Lanza N, Salbitani G, Basile A, Sorbo S, Vona V (2013) Physiological and morphological responses of Lead or Cadmium exposed Chlorella sorokiniana 211-8 K (Chlorophyceae). SpringerPlus 2:147. doi:10.1186/2193-1801-2-147
Chong AMY, Wong YS, Tam NFY (2000) Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 41:251–257. doi:10.1016/S0045-6535(99)00418-X
D’Souza L, Devi P, Divya Shridhar MP, Naik CG (2008) Use of Fourier transform infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina Tetrastromatica (Hauck). Anal Chem Insights 3:135–143
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627. doi:10.1016/j.biortech.2009.09.043
de-Bashan LE, Trejo A, Huss VAR, Hernandez J-P, Bashan Y (2008) Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol 99:4980–4989. doi:10.1016/j.biortech.2007.09.065
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Flouty R, Estephane G (2012) Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: a comparative study. J Environ Manage 111:106–114. doi:10.1016/j.jenvman.2012.06.042
Flouty R, Khalaf G (2015) Role of Cu and pb on Ni bioaccumulation by Chlamydomonas reinhardtii: validation of the biotic ligand model in binary metal mixtures. Ecotox Environ Safe 113:79–86. doi:10.1016/j.ecoenv.2014.11.022
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. doi:10.1016/j.cej.2009.09.013
Freundlich H (1926) Colloid and capillary chemistry. Methuen, London
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene: an overview. Environ Sci Pollut Res 20:2828–2843. doi:10.1007/s11356-013-1524-1
Gupta VK, Agarwal S, Saleh TA (2011a) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45:2207–2212. doi:10.1016/j.watres.2011.01.012
Gupta VK, Agarwal S, Saleh TA (2011b) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23. doi:10.1016/j.jhazmat.2010.08.053
Gupta VK, Jain R, Saleh TA, Nayak A, Malathi S, Agarwal S (2011c) Equilibrium and thermodynamic studies on the removal and recovery of Safranine-T dye from industrial effluents. Separ Sci Technol 46:839–846. doi:10.1080/01496395.2010.535591
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012a) Chemical treatment technologies for waste-water recycling-an overview. RSC Adv 2:6380–6388. doi:10.1039/c2ra20340e
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012b) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17. doi:10.1016/j.msec.2011.08.018
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interface Sci 193–194:24–34. doi:10.1016/j.cis.2013.03.003
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fund 5:212–223
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ 76:183–191
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ihsanullah et al (2015) Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J Mol Liq 204:255–263. doi:10.1016/j.molliq.2015.01.033
Kaewsarn P, Yu Q (2001) Cadmium(II) removal from aqueous solutions by pre-treated biomass of marine alga Padina sp. Environ Pollut 112:209–213. doi:10.1016/S0269-7491(00)00114-7
Kim S, J-e Park, Cho Y-B, Hwang S-J (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13. doi:10.1016/j.biortech.2013.06.068
Kumar K, Das D (2012) Growth characteristics of Chlorella sorokiniana in airlift and bubble column photobioreactors. Bioresour Technol 116:307–313. doi:10.1016/j.biortech.2012.03.074
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens, Handlingar 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Lizzul AM, Hellier P, Purton S, Baganz F, Ladommatos N, Campos L (2014) Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour Technol 151:12–18. doi:10.1016/j.biortech.2013.10.040
Mane PC, Bhosle AB (2011) Bioremoval of some metals by living algae Spirogyra sp. and Spirullina sp. from aqueous solution. Int. J Environ Res 6(2):571–576
Mehta SK, Gaur JP (2001) Removal of Ni and Cu from single and binary metalsolutions by free and immobilized Chlorella vulgaris. Eur J Protistol 37:261–271. doi:10.1078/0932-4739-00813
Monteiro C, Castro PL, Xavier Malcata F (2011) Biosorption of zinc ions from aqueous solution by the microalga Scenedesmus obliquus. Environ Chem Lett 9:169–176. doi:10.1007/s10311-009-0258-2
Onyancha D, Mavura W, Ngila JC, Ongoma P, Chacha J (2008) Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensata and Rhizoclonium hieroglyphicum. J Hazard Mater 158:605–614. doi:10.1016/j.jhazmat.2008.02.043
Pawar SN, Edgar KJ (2012) Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33:3279–3305. doi:10.1016/j.biomaterials.2012.01.007
Petrovič A, Simonič M (2015) The effect of carbon source on nitrate and ammonium removal from drinking water by immobilised Chlorella sorokiniana. Int J Environ Sci Technol 12:3175–3188. doi:10.1007/s13762-014-0747-0
Riaño B, Hernández D, García-González MC (2012) Microalgal-based systems for wastewater treatment: effect of applied organic and nutrient loading rate on biomass composition. Ecol Eng 49:112–117. doi:10.1016/j.ecoleng.2012.08.021
Rodrigues MS, Ferreira LS, Carvalho JCMd, Lodi A, Finocchio E, Converti A (2012) Metal biosorption onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris: multi-metal systems. J Hazard Mater 217–218:246–255. doi:10.1016/j.jhazmat.2012.03.022
Romera E, González F, Ballester A, Blázquez ML, Muñoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98:3344–3353. doi:10.1016/j.biortech.2006.09.026
Saleh TA (2011) The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl Surf Sci 257:7746–7751. doi:10.1016/j.apsusc.2011.04.020
Saleh TA (2015a) Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica- multiwall carbon nanotubes. Environ Sci Pollut Res 22:16721–16731. doi:10.1007/s11356-015-4866-z
Saleh TA (2015b) Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): from surface properties to sorption mechanism. Desalin Water Treat 57:10730–10744. doi:10.1080/19443994.2015.1036784
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interface Sci 362:337–344. doi:10.1016/j.jcis.2011.06.081
Saleh TA, Gupta VK (2012a) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371:101–106. doi:10.1016/j.jcis.2011.12.038
Saleh TA, Gupta VK (2012b) Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep Purif Technol 89:245–251. doi:10.1016/j.seppur.2012.01.039
Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv Colloid Interface Sci 211:93–101. doi:10.1016/j.cis.2014.06.006
Saleh TA, Agarwal S, Gupta VK (2011) Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl Catal B-Environ 106:46–53. doi:10.1016/j.apcatb.2011.05.003
Samuel J, Pulimi M, Paul ML, Maurya A, Chandrasekaran N, Mukherjee A (2013) Batch and continuous flow studies of adsorptive removal of Cr(VI) by adapted bacterial consortia immobilized in alginate beads. Bioresour Technol 128:423–430. doi:10.1016/j.biortech.2012.10.116
Sarı A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padina pavonica) biomass. J Hazard Mater 171:973–979. doi:10.1016/j.jhazmat.2009.06.101
Wan Maznah WO, Al-Fawwaz AT, Surif M (2012) Biosorption of copper and zinc by immobilised and free algal biomass, and the effects of metal biosorption on the growth and cellular structure of Chlorella sp. and Chlamydomonas sp. isolated from rivers in Penang Malaysia. J Environ Sci 24:1386–1393. doi:10.1016/S1001-0742(11)60931-5
Yoshida N, Ikeda R, Okuno T (2006) Identification and characterization of heavy metal-resistant unicellular alga isolated from soil and its potential for phytoremediation. Bioresour Technol 97:1843–1849. doi:10.1016/j.biortech.2005.08.021
Acknowledgments
The authors would like to acknowledge the Slovenian Research Agency (Javna Agencija za Raziskovalno Dejavnost RS) for their financial support (Project No. 1000-11-310131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrovič, A., Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana . Int. J. Environ. Sci. Technol. 13, 1761–1780 (2016). https://doi.org/10.1007/s13762-016-1015-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1015-2