Abstract
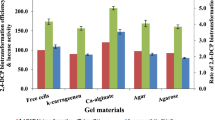
The biotreatment of industrial effluents that contain heavy metals, detergents and metal chelating agents represents a great concern to industries as these compounds are either toxic to microbes or they act as non-competitive inhibitors/denaturing agents for several enzymes. So, it is essential to know the effect of various agents usually present in the treatment plant on activity of the enzyme, for extending its applications in in situ treatment processes. This study describes the immobilization conditions of laccase and analysis of the sensitivity of immobilized laccase from Trichoderma viride Pers NFCCI-2745 to heavy metals, detergent and copper chelating agents. The concentrations and combinations of cations influenced the activity of immobilized enzyme and its yield. The immobilized Cu–Ba alginate enzyme showed comparatively higher activity compared to the Cu-alginate enzyme. Most of the metal ions tested enhanced laccase activity at lower concentration. Immobilized laccase retained more than 70 % of its activity even in the presence of 20 mM copper chelating agents like EDTA and sodium thioglycolic acid. Sodium azide, on the other hand, inhibited 80 % of the activity of all the beads even at a very low concentration of 0.5 mM. Cu–Ba and Cu–Ni alginate enzyme exhibited comparatively higher tolerance than Cu-alginate beads to EDTA and sodium thioglycolate. Tween 20 and cetyl trimethyl ammonium bromide significantly increased the activity of all the beads. These properties of the immobilized laccase may be aptly considered and suitably utilized in treating phenolic effluents that may contain heavy metals, detergents and certain copper chelating agents which may otherwise pose a threat to enzymatic activity.





Similar content being viewed by others
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco PA, Guebitz GM (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66:3357–3362
Aryee AN, Simpson BK (2012) Immobilization of lipase from grey mullet. Appl Biochem Biotechnol 168(8):2105–2122
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–242
Baldrian P, Gabriel J (2002) Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol Lett 206:69–74
Baldrian P, in Der Wiesche C, Gabriel J, Nerud F, Zadrazil F (2000) Influence of cadmium and mercury on activities of ligninolytic enzymes and degradation of polycyclic aromatic hydrocarbons by Pleurotus ostreatus in soil. Appl Environ Microbiol 66(6):2471–2478
Brandi P, D’Annibale A, Galli C, Gentili P, Pontes ASN (2006) In search for practical advantages from the immobilisation of an enzyme: the case of laccase. J Mol Catal B Enzym 41:61–69
Champagne PP, Nesheim ME, Ramsay JA (2013) A mechanism for NaCl inhibition of Reactive Blue 19 decolorization and ABTS oxidation by laccase. Appl Microbiol Biotechnol 97(14):6263–6269
Couto RS, Sanroman MA, Hofer D, Gubitz GM (2004) Stainless steel sponge: a novel carrier for the immobilisation of the white rot fungus Trametes hirsuta for decolourization of textile dyes. Bioresou Technol 95:67–72
Cuoto S, Herrera JL (2006) Fungal laccases: biotechnology application. Biotechnol Adv 2:500–513
Delanoy G, Li Q, Yu J (2005) Activity and stability of laccase in conjugation with chitosan. Int J Biol Macromol 35:89–95
Divya LM, Prasanth GK, Sadasivan C (2013a) Elimination of estrogenic activity of thermal paper using laccase from Trichoderma sp NFCCI-2745. Appl Biochem Biotechnol 169:1126–1133
Divya LM, Prasanth GK, Sadasivan C (2013b) Potential of salt tolerant laccase producing strain of Trichoderma viride Pers NFCCI-2745 from estuary in bioremediation of phenol polluted environments. J Basic Microbiol. doi:10.1002/jobm.201200394. [Epub ahead of print]
Divya LM, Prasanth GK, Sadasivan C (2013c) Isolation of a salt tolerant laccase secreting strain of Trichoderma Sp NFCCI-2745 and optimization of culture conditions and assessing its potential in the treatment of saline industrial effluents. J Environ Sci (accepted). doi:10.1016/S1001-0742(12)60321-s
Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases phenoloxidases immobilized on different supports: a review. Enzyme Microb Technol 31:907–993
Fukuda T, Uchida H, Takashima Y, Uwajima T, Kawabata T, Suzuki M (2001) Degradation of bisphenol A by purified laccase from Trametes villsoa. Biochem Biophys Res Commun 284:704–706
Hemmat J, Mazaheriassadi M (2013) Optimization of reactive blue 19 biodegradatin by Panerochaete chrysosporium. Int J Environ Res 7(4):957–962
Knezevic Z, Bobic S, Milutinovic A, Obradovic B, Mojovic L, Bugarski B (2002) Alginate-immobilized lipase by electrostatic extrusion for the purpose of palm oil hydrolysis in lecithin/isooctane system. Process Biochem 38:313–318
Laufer Z, Beckett R, Minibayeva FP (2006) Co-occurrence of the multicopper oxidases tyrosinase and laccase in lichens in sub-order Peltigerineae. Ann Bot 98(5):1035–1042
Lu L, Zhao M, Wang Y (2007) Immobilization of laccase by alginate-chitosan microcapsules and its use in dye decolorization. World J Microbiol Biotechnol 23:159–166
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochem 60:551–565
Nayanashree G, Thippeswamy B (2014) Natural rubber degradation by laccase and manganese peroxidase enzymes of Pencillium chrysogenum. Int J Environ Sci Technol. doi:10.1007/S13762-014-0636-6
Niladevi KN, Jacob N, Prema P (2008) Evidence for a halotolerantalkaline laccase in Streptomyces psammoticus: purification and characterization. Process Biochem 43:654–660
Palmieri G, Giardina P, Bianco C, Scaloni A (1997) A novel white laccase from Pleurotus ostreatus. J Biol Chem 272:31301–31307
Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G (2000) Copper induction of laccase isozymes in the lignolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 66:920–924
Peralta-Zamora P, Pereira CM, Tiburtius ERL, Moraes SG, Rosa MA, Minussi RC, Durán N (2003) Decolorization of reactive dyes by immobilized laccase. Appl Catal B Environ 42:131–144
Phetsom J, Khammuang S, Suwannawong P, Sarnthima R (2009) Copper-alginate encapsulation of crude laccase from Lentinus polychrous Lev. and their effectiveness in synthetic dyes decolorizations. J Biol Sci 9(6):573–583
Rama R, Mougin C, Boyer F, Kollmann A, Malosse C, Sigoillot J (1998) Biotransformation of benzo[a]pyrene in bench scale reactor using laccase of Pycnoporus cinnabrinus. Biotechnol Lett 20:1101–1104
Sharma KK, Shrivastava B, Sastry VRB, Sehgal N, Kuhad RC (2013) Middle-redox potential laccase from Ganoderma sp.: its application in improvement of feed for monogastric animals. Sci Rep 3:1299–12308
Sole M, Muller I, Pecyna MJ, Fetzer I, Harms H, Schlosser D (2012) Differential regulation by organic compounds and heavy metals of multiple laccase genes in the aquatic hyphomycete Clavariopsis aquatica. Appl Environ Microbiol 78:4732–4739
Teerapatsakul C, Abe N, Bucke C, Kongkathip N, Jareonkitmongkol S, Chitradon L (2008) Dye decolorisation by laccase entrapped in copper alginate. World J Microbiol Biotechnol 24:1367–1374
Tischer W, Kasche V (1999) Immobilized enzymes: crystals or carriers? Trends Biotechnol 17(8):326–335
Tyagi S, Kumar V, Singh J, Teotia P, Bisht S, Sharma S (2014) Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int J Environ Res 7(4):957–962
Won K, Kim S, Kim KJ, Park HW, Moon SJ (2005) Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem 40:2149–2154
Xu F, Kulys JJ, Duke K, Li K, Krikstopaitis K, Deussen HJ, Abbate E, Galinyte V, Schneider P (2000) Redox chemistry in laccase catalysed oxidation of N-hydroxy compounds. Appl Environ Microbiol 66:2052–2056
Yinghui D, Qiuling W, Shiyu F (2002) Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett Appl Microbiol 35(6):451–456
Acknowledgments
Department of Biotechnology and Microbiology, Kannur University, India, is gratefully acknowledged for the facilities provided to conduct this study.
Conflict of interest
There are no conflicts of interest in the opinion given in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Divya, L.M., Prasanth, G.K. & Sadasivan, C. Assessing the tolerance of immobilized laccase from a salt-tolerant strain of Trichoderma viride Pers NFCCI-2745 to heavy metal ions, detergents and copper chelating agents. Int. J. Environ. Sci. Technol. 12, 3225–3234 (2015). https://doi.org/10.1007/s13762-014-0697-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0697-6