Abstract
A technology of obtaining nitrogen-enriched activated carbons from coniferous tree sawdust by direct activation of the precursor and physical activation with CO2 is described. The effect of activation time, pyrolysis temperature as well as modification with urea on the textural parameters, acid–base character of the surface and sorption properties of activated carbons has been tested. The resulting carbons were characterised by low-temperature nitrogen sorption and determination of the number of surface oxygen groups. The sorption properties of the materials obtained were characterised by nitrogen dioxide adsorption in dry and wet conditions. The final products were nitrogen-enriched microporous activated carbons of medium-developed surface, showing very diverse nitrogen content and acidic–basic character of the surface. The results obtained in our study have proved that through suitable choice of the activation and modification procedure of coniferous tree sawdust, activated carbons can be produced with high capacity towards nitrogen dioxide adsorption, reaching to 69 and 46 mg NO2/g in dry and wet conditions, respectively. The results of our study have also shown that the adsorption ability of carbonaceous adsorbents depends both on the method of preparation as well as on the textural parameters and acid–base properties of the adsorbents surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased emission of toxic gases determines a very important worldwide environmental problem. The group of the most environmentally detrimental species is nitrogen oxides, NO x ; therefore, continuous control and minimisation of NO x emission are an important issue. Moreover, it is well known that although a main component of NO x is NO, NO2 is much more harmful. It is formed in the atmosphere as a result of photochemical oxidation of NO and directly in automobiles engines where even up to 30 % of NO x can be emitted as NO2 (Pietrzak and Bandosz 2007). Deterioration of the natural environment has for a long-time stimulated different measures leading to decreasing the amount of contaminants reaching water, soil and air. So no wonder that many approaches to solve this problem have been proposed. A much promoted approach is to replace the old technologies by cleaner and more effective modern ones and the use of carefully selected and purified precursors. Much attention has been paid to hermetisation of industrial processes and strict control of generated exhaust gases, wastewater or solid waste. Another approach is based on removal or neutralisation of the contaminants produced and employment of such new technological solutions as adsorption, absorption or catalytic reduction (Zhang et al. 2009a, b, 2010a, b, 2012a, b, 2013).
Of greatest interest is the process related to mass exchange—adsorption (Bansal and Goyal 2005). In the adsorption processes, a wide range of sorbents has been used, both inorganic (silica gels (Chung and Chung 1998), zeolites (Jamil et al. 2011), porous glass (Rysiakiewicz-Pasek et al. 2004), molecular sieves (Kopaς 1999) and aluminium oxide (Borggaard et al. 2005) and organic activated carbon fibres (Park and Kim 2001), carbon nanotubes (Shaijumon and Ramaprabhu 2005), carbon blacks (Li and Jaroniec 1999), carbon molecular sieves (Lozano-Castello et al. 2005) and mesoporous ordered carbons (Gierszal et al. 2005). The widest range of application has been predicted for activated carbons (Nowicki et al. 2010a; Ismadji et al. 2005), especially those containing different heteroatoms—O, N, S, P, halogens and metal ions (Bandosz 2009; Feng et al. 2006; Puziy and Poddubnaya 1998; Zeng et al. 2004; Goscianska et al. 2012, Somy et al. 2009), whose presence significantly modifies physicochemical properties. However, the majority of activated carbons do not contain such admixtures, which is related to their lack or very low content in the precursor used for the production of activated carbons. The solution proposed was development of effective methods of modification of the activated carbons (such as oxidation or ammoxidation) permitting introduction of different functional groups into the activated carbon structures (Pietrzak et al. 2009; Pradhan and Sandle 1999; Moreno-Castilla et al. 2000; Aguilar et al. 2003; Przepiórski 2006).
Recently, much attention has been paid to enrichment of activated carbons in nitrogen functional groups. So far many interesting and effective methods of nitrogen introduction have been proposed. The most important is the reactions of carbon with ammonia, urea or amines, the use of rich in nitrogen plastics as carbon precursors and impregnation of carbons with solutions of amines of different order or covering the surface of activated carbons with a layer of nitrogen-containing polymer (Bashkova and Bandosz 2009; Hayashi et al. 2005; Laszlo et al. 2001; Khalil et al. 2012; Maroto-Valer et al. 2005; Chen et al. 2003). Applying the above methods, it is possible to obtain a wide gamut of carbonaceous materials of different contents of nitrogen and different types of functional groups containing it.
The main objective of this paper was to obtain a series of activated carbons from common pine sawdust and their physicochemical characterisation. Different preparation parameters were examined in order to evaluate the influence of the pyrolysis and activation conditions on the properties of the final product. Additionally, we checked the effect of nitrogen doping on the properties of the activated carbons. Finally, we tried to correlate the surface properties with the ability of the activated carbons to remove nitrogen dioxide in dry and wet conditions.
The study was carried out in Faculty of Chemistry, Adam Mickiewicz University in Poznań, Poland, in September–December, 2012.
Materials and methods
Samples preparation
The starting material was pine sawdust (S) in the form of cylindrical pellets of length 20 and 5 mm in diameter. Adsorbents were prepared according to procedures differing in the order of the technological processes: (I) reaction of the precursor with urea followed by direct activation (SUDA series) or by pyrolysis and physical activation (SUP6A and SUP7A series), (II) reaction with urea after pyrolysis process followed by physical activation (SP6UA and SP7UA series) and (III) reaction with urea after activation (SDAU, SP6AU and SP7AU series). The non-modified samples (SDA and SP6A, SP7A) served as references. The sample codes and the preparation details are outlined in the scheme presented in Fig 1.
The pyrolysis process (P) was carried out in a horizontal furnace (equipped with quartz tube), under a stream of argon with a flow rate of 170 ml/min. Twenty grams of sawdust pellets was placed in quartz boat and next heated (10 °C/min) from room temperature to the final pyrolysis temperature of 600 (P6) or 700 °C (P7). The final temperature sample was maintained for 60 min, and then the system was cooled down to the room temperature.
Pyrolysis products were subjected to physical activation (A) by carbon dioxide. This process was preformed at 850 °C, under a stream of carbon dioxide with a flow rate of 0.250 L/min, for 60 (A1) and 120 min (A2).
Some parts of raw sawdust pellets (S) as well as sawdust modified with urea (SU) were also subjected to one-step activation (without pyrolysis stage) also called direct activation (DA). This process was conducted in the same conditions (temperature, time and CO2 flow) as physical activation.
Incorporation of nitrogen (U). Urea was used as a reagent introducing nitrogen functionalities into the carbon structure. The samples were mixed with urea at the weight ratio of 1:1 and then oxidised with oxygen from air at 350 °C. The reaction proceeded in a glass reactor for 3 h. The obtained nitrogen-enriched carbons were washed with hot distilled water to remove the unreacted part of urea and dried at 110 °C.
Analytical procedures
The elemental analysis of the starting pine pellets, as well as all the samples obtained was performed on an elemental analyzer CHNS Vario EL III. Characterisation of the pore structure of activated carbons was performed on the grounds of low-temperature nitrogen adsorption–desorption isotherms measured on a sorptometer ASAP 2010. Surface area and pore size distribution were calculated by BET and BJH methods, respectively. Total pore volume and average pore diameter were determined as well. Micropore surface area and volume were calculated using t-plot method.
The pH of the materials obtained was measured according to the procedure described earlier (Nowicki et al. 2012). Briefly, a portion of 0.5 g the sample of dry powder was added to 20 mL of demineralised water and the suspension was stirred overnight to reach equilibrium. After that the pH of the slurry was measured. The total content of surface functional groups of acidic and basic character was determined according to Boehm method (Boehm et al. 1964).
Adsorption studies
Evaluation of NO2 sorption capacity. Adsorption capacity towards NO2 was evaluated according to the procedure similar to that described elsewhere (Pietrzak and Bandosz 2007). The samples (1–2 mm particle size) were packed into a glass column (bed volume 3 cm3). Dry (“D”) or moist (70 % humidity) air (“W”) with 0.1 % of NO2 was passed through the bed of the adsorbent at 0.450 L/min for NO2. The breakthrough of NO2 and the concentration of NO were monitored using Q-RAE PLUS PGM-2000/2020 with electrochemical sensors. The tests were stopped at the breakthrough concentration of 20 ppm (because of electrochemical sensor limit). The capacities of each sorbent (in mg NO2/gads) were calculated by integration of the area above the breakthrough curve, and from the nitrogen dioxide concentration in the inlet gas, flow rate, breakthrough time and mass of adsorbent. All adsorption tests were made in triplicate, using a new portion of the carbon sample each time.
Results and discussion
Elemental composition of the samples obtained
Elemental analysis of the activated carbons obtained (Tables 1, 2, 3) has shown that their elemental composition depends to a significant degree on the order of reaction with urea, pyrolysis and activation processes as well as on the variant of activation procedure. Samples SP6AX and SP7AX (where X is the time of activation) obtained by physical activation of char SP6 and SP7, not subjected to modification with nitrogen, have the highest content of Cdaf and simultaneously the lowest content of oxygen, hydrogen and nitrogen (Table 3). Sample SDAX obtained by DA of precursor and samples SUDAX obtained from the precursor modified with urea were found to have similar elementary composition (Table 3) (Table 1). The lowest content of Cdaf and the highest contribution of the other elements were noted for samples SDAXU, SP6AXU and SP7AXU (Table 3) subjected to reaction with urea after activation. The content of particular elements depends also on the time of activation and for the samples obtained by a two-step activation—on the pyrolysis temperature. The influence of these parameters is much less important than that of the sequence of technological processes applied to the precursor.
According to the results obtained, the sequence in which the precursor (sawdust) was subjected to pyrolysis, activation and modification with nitrogen determines to a great degree of the amount of nitrogen introduced into the carbon structure. The content of this heteroatom in the obtained activated carbon samples varied in a wide range, from 0.2 wt% in SUDA1 and SUDA2 (Table 1) to 9.2 wt% in SDA2U (Table 3). So great differences in the content of Ndaf between the samples subjected to the reaction with urea at the stage of precursor (SUDAX, SUP6AX, SUP7AX—Table 1) and those subjected to this reaction at the stage of chars (SP6UAX, SP7UAX—Table 2), and the samples modified after activation (SDAXU, SP6AXU and SP7AXU—Table 3) are most probably a consequence of a low thermal resistance of nitrogen groups introduced into the carbon structure. Earlier studies by XPS (Pietrzak et al. 2006; Nowicki et al. 2010b; Burg et al. 2002) have proved that the reaction of carbonaceous materials with urea or ammonia involves generation of considerable amounts of amine, amide, nitrile, imine and lactam groups, characterised by low thermal stability. An indirect evidence of formation of such groups is a simultaneous increase in the content of nitrogen and hydrogen after the reaction with urea.
As follows from the data presented in Tables 1, 2, 3, the greatest amount of nitrogen (17.0 wt%) was introduced upon modification of precursor (Table 1). It should be noted that the efficiency of this process is considerably higher than that of analogous modification of bituminous coal (Pietrzak et al. 2009), although the particle sizes of the coal were much smaller. Moreover, the amount of nitrogen introduced into the structure and on the surface of sawdust was close to that obtained upon modification of fossil coal by gas ammonia which is a more effective nitrogenising agent (Nowicki et al. 2009). This evidence proves high reactivity of sawdust pellets towards urea. Much less nitrogen (4.6 and 6.8 wt%, respectively) was introduced into the structure of chars SP6 and SP7 (Table 2), which is related to a decrease in the susceptibility to chemical modification caused by the structure ordering upon pyrolysis (Nowicki et al. 2009, 2008). Unfortunately, a significant amount of nitrogen functional groups introduced into the precursor and chars underwent decomposition upon further thermal treatment as evidenced by a pronounced decrease in Ndaf after pyrolysis (SUP6 and SUP7 samples—Table 1), and in particular after activation (SUDAX, SP6UAX and SP7UAX series—Table 1). According to the data from this table, DA of the modified precursor (SU) resulted in total removal of the earlier introduced nitrogen groups. This fact confirms the earlier supposition of low thermal stability of nitrogen groups and points to a very high reactivity of modified precursor towards the activating agent—carbon dioxide. Less pronounced changes in the content of nitrogen were noted as a result of activation of samples SP6U and SP7U (Table 2), but the greatest amount of nitrogen (from among the samples modified prior to activation) was left in the structure of SUP6AX and SUP7AX (Table 1). It is probably a consequence of the fact that a considerable amount of nitrogen groups upon pyrolysis underwent transformation to more stable forms (Kapteijn et al. 1999; Cagniant et al. 1998) which meant that their content in the products of activation was much higher than in the other activated carbons.
The content of nitrogen in the samples subjected to the reaction with urea after activation varied in the range 7.5–9.2 wt% (Table 3), so it was a little higher than in samples SUP6AX and SUP7AX (Table 1). Efficiency of nitrogenation at this stage is about twice smaller than during precursor modification, but it is much higher than for chars SP6 and SP7. This result is probably a consequence of the presence of numerous oxygen groups on the surface of activated carbons which favours introduction of nitrogen groups (Pietrzak et al. 2006; Bimer et al. 1998).
Textural studies of activated carbons
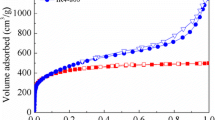
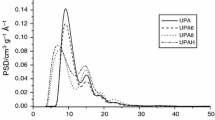
The values of surface area measured for the activated carbon samples, presented in Table 4, clearly illustrate a considerable influence of the activation method and time, temperature of pyrolysis and reaction with urea on the porous structure of the final product. The method of activation and the sequence in which the precursor was subjected to particular technological processes definitely have the greatest effect on the surface area. The samples obtained by DA of precursor have much more developed surface area and porous structure than the samples produced by the two-stage method. The greatest surface areas of 759 and 632 m2/g as well as the greatest total pore volumes of 0.40 and 0.37 cm3/g were found for SDA2 and SDA1 samples, respectively. Less beneficial textural parameters were determined for samples SP6AX and SP7AX obtained by the activation of unmodified chars SP6 and SP7 and samples SP6UAX and SP7UAX obtained by the activation of chars enriched in nitrogen after pyrolysis. The porous structure of carbon samples subjected to the reaction with urea at the stage of precursor, in particular those obtained by a two-step activation (except for sample SUP7A2) was even poorer. The poorest textural parameters were found for the samples subjected to the reaction with urea after the process of activation. Introduction of nitrogen at this stage caused a significant decrease in the surface area and the total pore volume, especially in samples SP6AXU and SP7AXU. The surface area of these samples varies from 23 to 70 m2/g, while the total pore volume is just 0.03–0.08 cm3/g. Most probably, such poor results follow from the fact that the nitrogen groups introduced upon modification have blocked the smallest pores playing the most important role in the porous structure of the carbon samples. This supposition is based on a considerable increase in the mean pore diameter from about 2.1–2.3 nm to even 5.25 nm and a drastic decrease in the contribution of micropores in the total pore volume.
Much less significant are the changes in the textural parameters of samples SDA1U and SDA2U as follows from the fact that their surface area is twice smaller than that of the initial samples SDA1 and SDA2. Similarly as for SP6AXU and SP7AXU, a decrease in the contribution of micropores and an increase in the mean pore diameter are observed, but the scale of these changes is much smaller.
In the samples of activated carbons enriched in nitrogen at the stage of precursor and chars, the contribution of micropores is much greater and varies from 80 to 96 %. The greatest contribution of micropores was found for samples SP6UAX and SP7UAX, in which micropores made 95–96 % of all pores and the mean pore diameter did not exceed 1.95 nm. The smallest contribution of pores of diameters below 2 nm (80 and 84 %, respectively) was established for samples SUP7A1 and SUP6A2 also showing the least beneficial textural parameters from among all samples subjected to the reaction with urea prior to activation.
A significant effect on the specific surface area and total pore volume of the carbon samples also has the time of activation. As indicated by the data presented in Table 4, the majority of samples activated for 2 h (except for SUP6A2) have better textural parameters than the materials subjected to activation for 1 h. It means that for the precursor used and for the chars obtained by its pyrolysis, the variant of shorter activation proved insufficient for effective development of porous structure. A comparison of the textural parameters of activated carbons obtained by the two-stage activation reveals some influence of the temperature of pyrolysis on the specific surface area and total pore volume. The carbon samples obtained by the activation of chars SUP6, SP6 and SP6U chars have somewhat greater SBET and V t than the samples obtained from chars SUP7, SP7 and SP7U. The differences are supposed to follow from the fact that a higher pyrolysis temperature favours greater ordering of the carbon structure, which makes it less reactive to the activating agent.
The particle size of the precursor and chars obtained by its pyrolysis, also influences the textural parameters of the activated carbon samples. As mentioned in the experimental section, the precursor (sawdust) was used in the form of pellets of relatively large size, which could significantly hinder the accessibility of deeper layers of carbon structure to carbon dioxide. Because of the use of large pellets, the surface areas and total pore volumes of the activated carbon samples are much less developed than those of the other carbon samples obtained by physical activation of sawdust (Ismadji et al. 2005; Tancredi et al. 1996).
Acid–base properties of activated carbons
In order to characterise the chemical properties of the surfaces of the activated carbon samples obtained, the contents of the surface oxygen functional groups of acidic and basic types as well as pH were measured. The data collected in Table 5 imply that the activated carbon samples obtained show much different acid–base properties. The pH of their surface changes from 7.0 to 10.4, while the total content of the surface oxygen groups varies from 0.10 to 2.20 mmol/g. The acid–base character of the surfaces of the samples obtained depends significantly on the method and time of activation, pyrolysis temperature and the stage at which the modification with urea was performed. From among the carbon samples modified with nitrogen at the stage of precursor, much higher content of oxygen groups was found in samples SUDA1 and SUDA2—obtained by DA. From among the samples subjected to the reaction with urea, followed by pyrolysis and activation, samples SUP6A1 and SUP6A2 were found to contain more acidic groups, while the analogous samples SUP7A1 and SUP7A2 had a higher content of basic groups. Similarly, dependencies were observed for the carbon samples modified with nitrogen at the stage of chars, so samples SP6UAX and SP7UAX. The difference between the two series of samples is much greater than that between samples SUP6AX and SUP6AX.
The highest pH (from 9.8 to 10.4) was measured for the samples obtained by DA of the precursor and activation of char SP6 not subjected to the reaction with urea at any stage. Lower pH values were measured for the carbon samples subjected to enrichment in nitrogen at the stage of precursor or chars, particularly those activated for 2 h. The lowest pH (7–8) was found for samples SP6AXU and SP7AXU, subjected to the reaction with urea after the activation.
The amount and types of oxygen functional groups on the surface of the carbon samples change in an irregular and complex way so it is difficult to find a general correlation between the variants of carbon obtaining and the content of acidic or basic groups. The highest content of oxygen groups (2.20 mmol/g) and the greatest contribution of basic groups (1.35 mmol/g) from among all samples obtained were found for sample SUDA2. Much less surface oxygen groups and also a distinct prevalence of basic groups were measured for samples SDA1, SP6AX, SUP7AX and SP7UAX, which contained from 4 to 9 times greater basic than acidic groups. On the other hand, samples SUDA1, and in particular SP6UA1 and SP6UA2, contain about 2–5 times more groups of acidic than those of basic character. The other samples show an intermediate acid–base properties and contain comparable amounts of acidic and basic groups.
Nitrogen dioxide adsorption properties
Table 6 data show that the adsorption capacities of the activated carbons obtained towards nitrogen dioxide differ depending on many factors: the sequence of individual processes (reaction with urea, pyrolysis and activation), the temperature of pyrolysis, the type and time of activation, as well as the conditions of adsorption. Almost all activated carbons show much greater sorption capacities when adsorption takes place in dry conditions. The most probable reason for poorer sorption properties of activated carbons in the wet conditions can be the competitive adsorption of steam. Only for samples SP6AXU and SP7AXU, the sorption capacity did not depend on the conditions of adsorption, but their sorption capacities were very small and did not exceed 4 mg NO2/gads. The most effective adsorbents were SDA2 and SP6A2, whose NO2 breakthrough capacities were 69 and 62 mg/g in dry conditions as well as 46 and 43 mg/g in wet conditions, respectively. Also high and close to 60 mg NO2/g was the sorption capacity of samples SP6UA2, SP7UA2, SUP7A1 and SDA1 in dry conditions. None of the samples enriched in nitrogen proved more effective than samples SDA2 and SP6A2. Particularly, low sorption capacity was measured for samples SDA1U and SDA2U subjected to the reaction with urea after the DA. Their sorption capacities were about three times smaller than those of the initial materials. Samples SP6A1U, SP6A2U, SP7A1U and SP7A2U shown practically no sorption abilities towards nitrogen dioxide. The so poor sorption capacities are related to first of all of their poor textural parameters and very low content of oxygen surface groups.
A comparison of the sorption capacities of particular series of adsorbents reveals that the most beneficial samples are those obtained by modification and DA of the precursor. From the economical point of view, this fact is advantageous as it permits reduction in the energy cost so also the cost of adsorbent production. It should be emphasised that these materials show sorption properties comparable with those of activated carbons obtained by high-temperature processing and modification of commercial materials based on wood or coal, or obtained by chemical activation of organic origin wastes. The latter process is expensive and generates large volumes of noxious wastes, related, e.g., with washing of the final product after activation. For the carbons obtained by the two-stage activation of great significance is the temperature of pyrolysis as evidenced by distinct differences in sorption capacities between the samples subjected to pyrolysis at 600 (P6) and 700 °C (P7). Also the time of activation influences the efficiency of nitrogen dioxide removal by the activated carbon samples obtained. For the samples not enriched in nitrogen at any stage and for those enriched in nitrogen at the stage of chars, a longer time of activation improves the sorption capacities towards NO2. This observation should probably be related to the fact that the carbon samples activated for 2 h have more beneficial textural parameters than those activated for 1 h. The opposite relation was found for the samples subjected to the reaction with urea at the stage of precursor. Such behaviour of the latter samples can follow from the facts that the samples activated for 1 h contain somewhat less surface oxygen groups than those activated for 2 h. Moreover, the above observation suggests that the presence of nitrogen in the carbon structure can influence to some extent the efficiency of nitrogen dioxide removal. Verification of this supposition needs further studies.
The results of sorption studies are also illustrated by the NO2 breakthrough curves (Fig. 2), and NO emission curves (Fig. 3) obtained during adsorption in dry and wet conditions. The character of the breakthrough curves changes in some degree depending on the variant of carbon samples obtaining and conditions of adsorption, which confirms the diversity of sorption capacities towards NO2 of the activated carbon samples. The curves recorded upon NO2 adsorption in dry conditions are similar for the majority of samples, which suggests a similar mechanism of NO2 adsorption. For the majority of samples, especially for SDA2 D, SP6A2 D, SP6UA2 D and SP7UA2 D, for a long time, the NO2 concentration recorded is close to zero and the adsorption curve is parallel to the x axis. Only for the carbon samples subjected to the reaction with urea after activation (SP6A1U D, SP6A2U D, SP7A1U D and SP7A2U D), the so-called breakthrough of adsorbents bed takes place after a very short time and then a rapid increase in the NO2 concentration to 20 ppm is observed. As indicated by the “desorption” fragment of the curves, when the supply of NO2 to the system is cut-off, a rapid decrease in the NO2 concentration is observed, which suggests that the majority of NO2 adsorbed has been strongly bound to the surface or structure of the activated carbon, probably by chemisorption (Kaźmierczak et al. 2013). For samples SDA1 D, SDA2 D, SP6A2 D, SP6UA2 D, SP7A2 and SP7UA2 D, the concentration of NO2 after 30 min of desorption was above 10 ppm, which means that a large part of the NO2 adsorbed has been loosely bound to the carbon surface and is released upon washing out with a flux of air. It suggests that in the latter samples, NO2 undergoes mainly physisorption.
A comparison of the shapes of NO2 breakthrough curves obtained during adsorption in dry and wet conditions (Fig. 2) shows that for the majority of samples, no significant differences in the character of breakthrough curves appeared after introduction of water, which suggests a similar mechanism of nitrogen dioxide adsorption irrespective of the conditions. The greatest differences in the breakthrough curves were noted for the carbon samples obtained by DA (in particular SUDA1 W) and for sample SP6UA2 W obtained by physical activation of nitrogen-enriched char SP6.
Analysis of the “desorption” parts of the curves implies that similarly as in dry conditions, the majority of NO2 adsorbed in wet conditions is permanently bound to the structure or surface of activated carbon samples. This conclusion is based on observations of very fast decrease in the NO2 concentration after cutting off its supply to the system. Thus, it can be supposed that adsorption in wet conditions has mainly chemical character. The supposition is based on the fact that the presence of water can lead to formation of HNO3 as well as HNO2, which favour effective adsorption of nitrogen dioxide on the surface of the adsorbents (Pietrzak and Bandosz 2008).
As follows from the data presented in Fig. 3, the activated carbon samples studied besides the sorption abilities towards NO2 also show the ability to NO2 reduction as evidenced by considerable amounts of nitrogen monoxide accompanying the process of NO2 adsorption. Efficiency of NO2 reduction in NO is much different for different samples and significantly depends on the conditions of adsorption. In general, the majority of activated carbon samples show much greater reduction potential when NO2 adsorption takes place in wet conditions. The evidence supporting the above claim is that in the wet conditions, the time needed to reach NO concentration of 200 ppm (maximum value detectable by the sensor used in the studies) was much shorter than in dry conditions. It can also be noted that NO2 reduction was the most intensive for the carbon samples obtained by modification and DA of the precursor, while the smallest amounts of NO were generated by the sample obtained by modification of char SP6.
Analysis of the data collected also implies that the ability of the activated carbon samples studied to reduce NO2 can to a significant degree influence the efficiency of NO2 adsorption. A greater efficiency of NO2 reduction in wet conditions is probably the reason for the poorer adsorption abilities towards this gas. Large amounts of NO generated in the process of adsorption can compete with NO2 molecules for the access to the active centres of the carbon samples studied and thus deteriorate the sorption capacity. Verification of this supposition would require a more complex measuring setup which would permit accurate record of the processes taking place in the adsorbent bed.
Conclusion
The above discussed results permit drawing a few important conclusions concerning the preparation, physicochemical properties and sorption properties of nitrogen-enriched activated carbons obtained from pine sawdust pellets. Introduction of nitrogen groups at different stages of activated carbons production permitted getting a large gamut of materials showing diverse elemental composition and acid–base properties of the surface. It has been shown that by applying different sequences and variants of pyrolysis, activation and modification with urea, it is possible to obtain carbon samples of different development of surface area and more or less microporous character. Moreover, many of the activated carbon samples obtained show high sorption capacity towards nitrogen dioxide only in dry conditions. The majority of the carbon samples obtained show also high reduction potential of NO2, which has a negative influence on their sorption capacity towards this gas (especially samples SDA1, SDA2 and SP6A2).
Further investigation should be aimed at elimination of significant diminishing of the reduction potential of activated carbon towards nitrogen dioxide and thus at improvement of its adsorption capacity towards this gas in wet conditions.
References
Aguilar C, Garcia R, Soto-Garrido G, Arriagada R (2003) Catalytic wet air oxidation of aqueous ammonia with activated carbon. Appl Catal B-Environ 46:229–237
Bandosz TJ (2009) Surface chemistry of carbon materials. In: Serp P, Figueiredo JL (eds) Carbon materials for catalysis. Wiley, New Jersey, pp 45–92
Bansal RCh, Goyal M (2005) Activated carbon adsorption. Taylor & Francis Group, Boca Raton
Bashkova S, Bandosz TJ (2009) The effects of urea modification and heat treatment on the process of NO2 removal by wood-based activated carbon. J Colloid Interf Sci 333:97–103
Bimer J, Sałbut PD, Berłożecki S, Boudou JP, Broniek E, Siemieniewska T (1998) Modified active carbons from precursors enriched with nitrogen functions: sulfur removal capabilities. Fuel 77:519–525
Boehm HP, Diehl E, Heck W, Sappok R (1964) Surface oxides of carbon. Angew Chem Int Edit Engl 3:669–677
Borggaard OK, Raben-Lange B, Gimsing AL, Strobel BW (2005) Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma 127:270–279
Burg P, Fydrych P, Cagniant D, Nanse G, Bimer J, Jankowska A (2002) The characterization of nitrogen-enriched activated carbons by IR, XPS and LSER methods. Carbon 40:1521–1531
Cagniant D, Gruber R, Boudou JP, Bilem C, Bimer J, Salbut PD (1998) Structural characterization of nitrogen-enriched coals. Energy Fuel 12:672–681
Chen WC, Wen TC, Teng H (2003) Polyaniline-deposited porous carbon electrode for supercapacitor. Electrochim Acta 48:641–649
Chung TW, Chung CC (1998) Increase in the amount of adsorption on modified silica gel by using neutron flux irradiation. Chem Eng Sci 53:2967–2972
Feng W, Borguet E, Vidic RD (2006) Sulfurization of carbon surface for vapor phase mercury removal—I: effect of temperature and sulfurization protocol. Carbon 44:2990–2997
Gierszal KP, Jaroniec M, Kim TW, Kim J, Ryoo R (2005) High temperature treatment of ordered mesoporous carbons prepared by using various carbon precursors and ordered mesoporous silica templates. New J Chem 32:981–993
Goscianska J, Nowak I, Nowicki P, Pietrzak R (2012) The influence of silver on the physicochemical and catalytic properties of activated carbons. Chem Eng J 189–190:422–430
Hayashi J, Yamamoto N, Horikawa T, Muroyama K, Gomes VG (2005) Preparation and characterization of high-specific-surface-area activated carbons from K2CO3-treated waste polyurethane. J Colloid Interf Sci 281:437–443
Ismadji S, Sudaryanto Y, Hartono SB, Setiawan LEK, Ayucitra A (2005) Activated carbon from char obtained from vacuum pyrolysis of teak sawdust: pore structure development and characterization. Bioresource Technol 96:1364–1369
Jamil TS, Abdel Ghafar HH, Ibrahim HS, Abd El-Maksoud IH (2011) Removal of methylene blue by two zeolites prepared from naturally occurring Egyptian kaolin as cost effective technique. Solid State Sci 13:1844–1851
Kapteijn F, Moulijn JA, Matzner S, Boehm HP (1999) The development of nitrogen functionality in model chars during gasification in CO2 and O2. Carbon 37:1143–1150
Kaźmierczak J, Nowicki P, Pietrzak R (2013) Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 19(2):273–281
Khalil SH, Aroua MK, Daud WMAW (2012) Study on the improvement of the capacity of amine-impregnated commercial activated carbon beds for CO2 adsorbing. Chem Eng J 183:15–20
Kopaς T (1999) Non-isobaric adsorption analysis of SO2 on molecular sieve 13X and activated carbon by dynamic technique. Chem Eng Process 38:45–53
Laszlo K, Tombacz E, Josepovits K (2001) Effect of activation on the surface chemistry of carbons from polymer precursors. Carbon 39:1217–1228
Li Z, Jaroniec M (1999) Comparative studies of carbon blacks by thermogravimetry and nitrogen adsorption. J Colloid Interf Sci 210:200–206
Lozano-Castello D, Alcaniz-Monge J, Cazorla-Amoros D, Linares-Solano A, Zhu W, Kapteijn F, Moulijn JA (2005) Adsorption properties of carbon molecular sieves prepared from an activated carbon by pitch pyrolysis. Carbon 43:1643–1651
Maroto-Valer MM, Tang Z, Zhang Y (2005) CO2 capture by activated and impregnated anthracites. Fuel Process Technol 86:1487–1502
Moreno-Castilla C, Lopez-Ramon MV, Carrasco-Marin F (2000) Changes in surface chemistry of activated carbons by wet oxidation. Carbon 38:1995–2001
Nowicki P, Pietrzak R, Wachowska H (2008) Comparison of physicochemical properties of nitrogen-enriched activated carbons prepared by physical and chemical activation of brown coal. Energy Fuel 22:4133–4138
Nowicki P, Pietrzak R, Wachowska H (2009) Influence of metamorphism degree of the precursor on preparation of nitrogen enriched activated carbons by ammoxidation and chemical activation of coals. Energy Fuel 23:2205–2212
Nowicki P, Wachowska H, Pietrzak R (2010a) Active carbons prepared by chemical activation of plum stones and their application in removal of NO2. J Hazard Mater 181:1088–1094
Nowicki P, Pietrzak R, Wachowska H (2010b) X-ray photoelectron spectroscopy study of nitrogen-enriched active carbons obtained by ammoxidation and chemical activation of brown and bituminous coals. Energy Fuel 24:1197–1206
Nowicki P, Supłat M, Przepiórski J, Pietrzak R (2012) NO2 removal on adsorbents obtained by pyrolysis and physical activation of corrugated cardboard. Chem Eng J 195–196:7–14
Park SJ, Kim KD (2001) Influence of activation temperature on adsorption characteristics of activated carbon fiber composites. Carbon 39:1741–1746
Pietrzak R, Bandosz TJ (2007) Activated carbons modified with sewage sludge derived phase and their application in the process of NO2 removal. Carbon 45:2537–2546
Pietrzak R, Bandosz TJ (2008) Interactions of NO2 with sewage sludge based composite adsorbents. J Hazard Mater 154:946–953
Pietrzak R, Wachowska H, Nowicki P (2006) Preparation of nitrogen-enriched activated carbons from brown coal. Energy Fuel 20:1275–1280
Pietrzak R, Nowicki P, Wachowska H (2009) The influence of oxidation with nitric acid on the preparation and properties of active carbon enriched in nitrogen. Appl Surf Sci 255:3586–3593
Pradhan BK, Sandle NK (1999) Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 37:1323–1332
Przepiórski J (2006) Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J Hazard Mater 135:453–456
Puziy AM, Poddubnaya OI (1998) The properties of synthetic carbon derived from nitrogen- and phosphorus-containing polymer. Carbon 36:45–50
Rysiakiewicz-Pasek E, Vorobyova VA, Gevelyuk SA, Doycho IK, Mak VT (2004) Effect of potassium nitrate treatment on the adsorption properties of silica porous glasses. J Non-Cryst Solids 345–346:260–264
Shaijumon MM, Ramaprabhu S (2005) Studies of yield and nature of carbon nanostructures synthesized by pyrolysis of ferrocene and hydrogen adsorption studies of carbon nanotubes. Int J Hydrogen Energy 30:311–317
Somy A, Mehrnia MR, Amrei HD, Ghanizadeh A, Safari M (2009) Adsorption of carbon dioxide using impregnated activated carbon promoted by Zinc. Int J Greenh Gas Control 3:249–254
Tancredi N, Cordero T, Rodriguez-Mirasol J, Rodriguez JJ (1996) Activated carbons from Uruguayan eucalyptus wood. Fuel 75:1701–1706
Zeng H, Jin F, Guo J (2004) Removal of elemental mercury from coal combustion flue gas by chloride-impregnated activated carbon. Fuel 83:143–146
Zhang J, Sun J, Gong Y, Wang D, Ma T, Liu Y (2009a) A scheme for solving strongly coupled chemical reaction equations appearing in the removal of SO2 and NOx from flue gases. Vacuum 83:133–137
Zhang Z, Chen M, Shangguan W (2009b) Low-temperature SCR of NO with propylene in excess oxygen over the Pt/TiO2 catalyst. Catal Commun 10:1330–1333
Zhang D, Cai Q, Cong L (2010a) Enhancing completely autotrophic nitrogen removal over nitrite by trace NO2 addition to an AUSB reactor. J Chem Technol Biotechnol 85:204–208
Zhang L, Yang J, Furukawa K (2010b) Stable and high-rate nitrogen removal from reject water by partial nitrification and subsequent anammox. J Biosci Bioeng 4:441–448
Zhang C, Boudiba A, Navio C, Olivier MG, Snyders R, Debliquy M (2012a) Study of selectivity of NO2 sensors composed of WO3 and MnO2 thin films grown by radio frequency sputtering. Sens Actuators B Chem 161:914–922
Zhang LL, Wang JX, Sun Q, Zeng XF, Chen JF (2012b) Removal of nitric oxide in rotating packed bed by ferrous chelate solution. Chem Eng J 181–182:624–629
Zhang D, Li W, Huang X, Qin W, Liu M (2013) Removal of ammonium in surface water at low temperature by a newly isolated Microbacterium sp. strain SFA13. Bioresour Technol 137:147–152
Acknowledgments
This work was supported by The Polish Ministry of Science and Higher Education Project No. N N204 277537.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nowicki, P., Kazmierczak, J., Sawicka, K. et al. Nitrogen-enriched activated carbons prepared by the activation of coniferous tree sawdust and their application in the removal of Nitrogen dioxide. Int. J. Environ. Sci. Technol. 12, 2233–2244 (2015). https://doi.org/10.1007/s13762-014-0611-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0611-2