Abstract
Background
Extracorporeal membrane oxygenation (ECMO) in critically ill patients serves as a management option for end-stage cardiorespiratory failure in medical and surgical conditions. Patients on ECMO are at a high risk of neurologic adverse events including intracranial hemorrhage (ICH), acute ischemic stroke (AIS), seizures, diffuse cerebral edema, and hypoxic brain injury. Standard approaches to neurological monitoring for patients receiving ECMO support can be challenging for multiple reasons, including the severity of critical illness, deep sedation, and/or paralysis. This narrative literature review provides an overview of the current landscape for neurological monitoring in this population.
Methods
A literature search using PubMed was used to aid the understanding of the landscape of published literature in the area of neurological monitoring in ECMO patients.
Results
Review articles, cohort studies, case series, and individual reports were identified. A total of 73 varied manuscripts were summarized and included in this review which presents the challenges and strategies for performing neurological monitoring in this population.
Conclusion
Neurological monitoring in ECMO is an area of interest to many clinicians, however, the literature is limited, heterogenous, and lacks consensus on the best monitoring practices. The evidence for optimal neurological monitoring that could impact clinical decisions and functional outcomes is lacking. Additional studies are needed to identify effective measures of neurological monitoring while on ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Extracorporeal membrane oxygenation (ECMO), a type of extracorporeal circulatory life support (ECLS), is a form of mechanical cardiopulmonary support utilized for end-stage cardiorespiratory failure that is refractory to maximal medical therapy. Since its introduction by Gibbon in 1953, major advances have been made to its technique and application, allowing for prolonged survival of some of the most fatal medical and surgical conditions [1]. Standard approaches for evaluating the neurological status of these patients are challenging during ECMO support. In this narrative literature review, we provide an overview of these challenges and available strategies for performing a neurological assessment in this patient population.
Methods
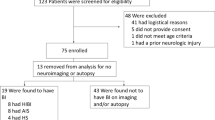
We performed a search in July 2021 using PubMed. The terms “ECMO”, “ECLS”, “Neurologic Monitoring”, “EEG”, “Neuroimaging”, “Transcranial Doppler”, “Somatosensory Evoked Potential”, “COVID-19” and “Neuromonitoring” were used either individually or in combination. The results of the search highlighted 73 relevant articles which were summarized by two critical care fellows and two neurology residents with oversight from a neuro-intensivist and two emergency medicine and critical care physicians who have expertise in ECMO.
REVIEW
ECMO: review of the types of support and indications for use
The two major configurations for ECMO support are veno-venous (V-V) and veno-arterial (V-A) ECMO [2]. V-A ECMO provides respiratory and circulatory support, most often for cases of cardiac arrest, refractory cardiogenic shock, right ventricular failure, as a bridge to cardiac transplantation and in the post-cardiotomy syndrome following cardiac bypass surgery. V-V –ECMO provides respiratory support and is used most often in cases of acute respiratory distress syndrome (ARDS) and severe hypercapnic respiratory failure.
Early data on its use involved small observational studies as well as uncontrolled trials that reported increased survival rates up to 50–71% compared to patients who did not receive ECMO [3,4,5,6]. During the 2009 H1N1 influenza A pandemic, the use of V-V ECMO provided a successful organ support for patients with ARDS, failing conventional ventilation, with survival rates of up to 68% [7]. Additionally, the “Conventional ventilatory support versus extracorporeal membrane oxygenation for severe acute respiratory failure (CESAR trial)” was conducted in 2009. It randomized 180 patients with severe ARDS to either referral to an ECMO center or continued conventional management. This trial found a difference in the 6-month survival rate between the patients referred to the ECMO center and control subjects (63 vs. 47%) and was terminated early due to its futility with findings favoring the use of ECMO support [8]. A major criticism of this trial was the heterogeneous ventilation strategies within the control subjects.
The ECMO to rescue lung injury in severe ARDS (EOLIA trial), published in 2018 examined 249 patients with severe ARDS. It randomized patients to receive early V-V ECMO support or conventional lung-protective ventilation with the use of low tidal volumes and low-pressure ventilation, with the possibility of crossover to ECMO for patients with refractory hypoxemia. They found a significant improvement in oxygenation and days free of renal failure (46 vs. 21%) with lower rates of ischemic stroke (IS) (0 vs 5%) in patients in the ECMO compared to the conventional therapy arm. This trial was also terminated early for futility with findings favoring the use of ECMO. Final analysis showed an 11% improvement in absolute mortality rates with ECMO compared to the control group. Many believe that the EOLIA trial supports early ECMO use in severe ARDS not responding to optimum management strategies involving lung protective ventilation, pulmonary vasodilators, prone ventilation and the use of neuromuscular blockers [9].
Thiagarajan et al. examined data from the extracorporeal life support registry organization (ELSO) from 1989 to 2016, analyzing the use of extracorporeal life support (ECLS) and survival rates. They reported survival to discharge rate of 58% in 78,397 patients who received ECLS [10]. A systematic review of practices and outcomes following V-A ECMO resuscitation for refractory out-of-hospital cardiac arrest in adult patients identified a 22% survival rate amongst the 822 patients who received eCPR. Of the survivors, 13% had good neurological outcomes [11]. More recently in the ARREST (Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation) trial, 30 patients with out-of-hospital cardiac arrest were randomized to eCPR versus standard advanced cardiac life support. The study was terminated early because the superiority of the ECMO arm exceeded the preplanned endpoint with 43% survival to hospital discharge in the ECMO group versus 7% in the control group [12].
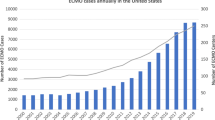
These shifts propelled the utilization of ECMO for organ support and the number of centers providing ECMO have grown exponentially [13].
Neuromonitoring and challenges in ECMO patients
Neurological disturbances impacting patients receiving ECMO may occur during the initiation of ECMO support and as a complication of ongoing ECMO support. Thus, the neurological assessment of the patient is paramount yet presents challenges.
CO2 reactivity during ECMO
Carbon Dioxide (CO2) and pH play a significant role and are quite critical in the regulation of cerebral blood flow [14]. Having that said, the abrupt reduction in CO2 blood levels during the initiation of ECMO Support can facilitate complications including intracranial hemorrhage [15]. The pathophysiological mechanism attributed to this is blood vessels vasoconstriction resulting from hypocapnia. This in turn results in an ischemic infarct and hence, hemorrhagic conversion. This is also precipitated by the systemic anticoagulation frequently used while on ECMO support [16].
Physical examination
The 2017 ELSO guidelines recommend sedation for at least the first 12–24 h of ECMO initiation to prevent air embolism from spontaneous respirations as well as for patient comfort [17]. Thus, the sensitivity of the neurologic exam in patients on ECMO is often compromised due to the use of sedatives, analgesics and sometimes paralytics [18, 19]. The combined central nervous system (CNS) depression due to sedatives and analgesics reduces the level of consciousness and precludes the assessment of higher cortical function. Additionally, deeper levels of pharmacologic CNS depression impair brainstem reflexes. One study reports 24% of patients suffering from neurologic complications while on ECMO had no clinical neurological signs prior to neuroimaging [18].
Neuroimaging
Obtaining neuroimaging to investigate suspected neurological disturbances in ECMO patients poses a challenge. From a technical perspective, contrast-based imaging modalities can be particularly misleading due to ECMO [19, 20]. Logistically, the severity of the critical illness and the degree of mechanical support in patients supported by ECMO poses a challenge for safe transport to radiological testing areas [18]. The instability of the underlying cardiorespiratory illness often requires the availability of multiple team members including respiratory therapists and perfusionists for safe transport to an imaging suite.
Non-contrast computed tomography
Non-contrast computed tomography (NCCT) is a comparably feasible, relatively safe and cost-effective method of neurologic assessment in ECMO patients [18, 21]. Lidegran et al. reviewed 123 consecutive cases of adult and pediatric ECMO patients over a ten-year period. They identified neurologic complications confirmed by NCCT-influenced treatment, ranging from adjusting anticoagulation to weaning ECMO for withdrawal of care in cases of catastrophic insults (ex. diffuse cerebral edema) [18]. In patients sustaining a survivable insult such as focal hemorrhagic or AIS adjustments were made in systemic anticoagulation intensity to facilitate continued ECMO support [18]. No specific lateralization of neurological injury on NCCT was noted in relation to the side of cannulation [18]. LaRovere et al. showed that 41% of patients treated with ECMO had bilateral infarctions, however, the unilateral injuries showed no relation to the side of cannulation [22].
CT angiography, CT perfusion and conventional cerebral angiograms
Limited studies identify challenges facing contrast-based neuroimaging in ECMO patients including computed tomography angiography (CTA) of the head and neck, CT perfusion (CTP) and conventional cerebral angiograms (CCA) [19, 20]. This can become an issue with distinct sites of V-AECMO cannulation including outflow cannulas placed in the common iliac or axillary arteries. In our experience cannulation through the axillary artery presents an issue of high-pressure non-opacified blood flow competing with the systemic contrast-opacified blood causing unilateral non-opacification of the extracranial and intracranial vessels. The CTA and CTP resulted in false positives mimicking LVO with irreversibly infarcted tissue, respectively [19]. CCA can demonstrate the reversal of flow in the brachiocephalic and subclavian arteries in patients with right axillary cannulation [19]. Another group has shown similar disturbances of hemodynamics from V-A ECMO where the outflow cannula is in the common iliac artery. Acharya et al. described a case where a draining cannula was placed near the right atrium and the return cannula was placed in the common iliac artery. As the contrast is injected intravenously, the draining (venous cannula) will siphon the opacified blood through the ECMO circuitry. The contrast-opacified blood will flow retrogradely in the descending aorta (V-A ECMO) and hence, will compete with the un-opacified blood flowing antegrade from the left ventricle into the aortic arch. The opacified blood will preferentially flow into the left subclavian and left common carotid arteries while the non-opacified blood will preferentially flow into the right brachiocephalic and common carotid arteries giving rise to asymmetric contrast opacification of extracranial and intracranial vasculature on CTA [20].
Magnetic resonance imaging
MRI remains an excellent way of identifying neurologic complications such as cerebral microbleeds (CMBs) after ECMO decannulation. CMBs have a distinct distribution in adults managed with either V-A or V-V ECMO, being mostly limited to the subcortical U fibers and deep white matter compared to the more lobar distribution in cerebral amyloid angiopathy and deep grey matter in small vessel arteriopathy [23, 24]. The authors, however, did not mention whether baseline MRIs were available for comparison with MRIs after ECMO. Interestingly, a different pattern was noted in pediatric patients treated with V-A ECMO where CMBs were identified in the watershed areas in the internal carotid artery ipsilateral to the cannulation side. This may be from hypoperfusion secondary to the ECMO cannula being placed in the ipsilateral ICA or from small embolic particles such as air emboli. However, CMBs in both populations were asymptomatic [25]. Cho et al. in the (SAFE-MRI ECMO) study, found that low-field portable MRI is a safe and logistically feasible option in ECMO patients. It also offers a diagnostic advantage over NCCT [26]. However, this remains hindered by the limited availability of portable MRI at most institutions currently. A further challenge to MRI accessibility is that the ECMO machinery itself can be incompatible with the MRI magnet [27].
Other ancillary testing
The physical examination and standard neuroimaging modalities have challenges and limitations in ECMO patients. Ancillary neuromonitoring modalities are available with greater accessibility at the bedside.
Transcranial Dopplers–TCD–Supplemental Table 1
Transcranial Dopplers (TCDs) are a useful non-invasive bedside modality for cerebral hemodynamic monitoring [28]. The data behind the use of TCDs in patients with cerebrovascular disease is vast and well-established, dating back to the 1990s. Measurements obtained with TCDs provide helpful information for evaluating cerebral autoregulation through the patterns of mean flow velocities (MFV) and pulsatility indices (PI). However, not all institutions have access to TCDs, and implementation of a TCD program requires an experienced technician and vascular neurologist for acquisition and interpretation, respectively.
Applications of TCD in pediatric ECMO population
A retrospective review of 27 pediatric patients on ECMO investigated the association between cerebral blood flow velocities and neurological injury, using TCDs. Although Rilinger et al. found that mean, systolic and diastolic blood flow velocity increased after ECMO decannulation, there was no association found between cerebral blood flow velocities and the presence of neurological abnormalities. An interesting finding from this study, however, was neonates on ECMO were found to have higher velocities than expected when compared to age-matched controls [29].
A 2019 study of 52 V-A ECMO patients showed that in the 13 patients that suffered a neurologic injury (electrographic seizures, AIS or ICH), the middle cerebral artery (MCA) PI was significantly higher compared to those not suffering from neurologic injury. However, there was no side-to-side difference in blood flow velocities. Patients not suffering from neurologic injury have lower than predicted systolic and MFV as well as PIs in the first few days following cannulation. This in part could be secondary to heavier sedation in the first few days following cannulation. There was no difference in diastolic flow velocities of their entire cohort compared with the predicted normal. The lack of pulsatility attributed to V-A ECMO support, adds some uncertainty to the interpretation of PIs. Although there was a greater than 30% side-to-side difference in systolic flow velocities in peripherally cannulated patients, none of them suffered neurologic injury. However, the authors suggested that a persistent side to side difference should trigger sedation cessation for a better neurologic exam [30].
In a 2013 study of TCDs in ECMO patients, four out of eighteen patients suffered from ICH across the V-A and V-V ECMO groups, the velocities including systolic, diastolic, and mean exceeded predicted values by 23, 30 and 27%, respectively. The elevated velocities were noted between 2 and 6 days before the clinical event. Additionally, in those four patients, the Lindergardt ratio was lower than 3 suggesting the presence of hyperemia at the time of the TCD and the underlying etiology of ICH [31].
Applications of TCD in adult ECMO population
In 2010, Zanatta and colleagues studied 6 patients undergoing V-A ECMO using TCDs for micro embolic signal (MES) detection. There were 4 patients treated with 100% blood flow support on V-A ECMO and all had MES detected by TCD. However, the other 2 patients were treated with blood flow support of 50% of normal flow requirements and had no MES [32]. In a similar study on MES detection, Marinoni et al. divided MES detection into mild, moderate and severe groups depending on the number of signals counted during a 60-min continuous TCD as 1–20, 21–99, and ≥ 100 MESs, respectively. In the V-V ECMO group 26.2% had no MESs, and none saw ≥ 100. MESs were detected in 81.8% of their V-A group, 45.4% of which had ≥ 100. Consistent with this pattern, was the higher number of MESs detected in patients with lower left ventricular ejection fraction. There was no difference shown in the laterality of the MES. Also no correlation was seen between MESs and coagulation values, raises the likelihood that air emboli comprise the majority of MESs [33]. On a similar note, Min Cho et al. showed that no V-V ECMO patients suffered from MESs. However, MES was detected in 47% of the V-A group and 77.7% of the embolic events occurred in this group. Interestingly, MES was noted across therapeutic and non-therapeutic aPTT levels (29.4 and 18%, respectively) [34].
V-A ECMO patients with severely decreased cardiac function also have the added feature of reduced or lost pulsatility of blood flow [28, 35]. One study found that PIs directly correlate with the severity of myocardial suppression with loss of pulsatility in patients with EF < 10% and should not be confused for cerebral vasodilation. Another study showed that pulsatility was lost with EF as low as 20%, and rising PI coincided with an improvement in myocardial function. PIs typically normalized following ECMO decannulation [28, 36].
In a systematic review of neuromonitoring in adult and pediatric ECMO patients, PIs were inversely associated with ECMO flow rates. An increased CBF with a low PI at high ECMO flow rates may suggest a hyper-perfusion injury mechanism contributing to the development of ICH [37].
Yang et al. evaluated the effect of IABP on CBF, as measured at bilateral MCAs, by TCDs during V-A ECMO support. The addition of IABP in post-cardiac surgery patients showed either increased CBF in those without stunned myocardium (pulsatile pressure > 10 mmHg) or reduced CBF in those with it (pulsatile pressure < 10 mmHg). This adds more value to the importance of CBF monitoring during ECMO by TCDs [38].
Despite conflicting statements in the Salna et al. comment that in V-A ECMO patients cannulated through either axillary or femoral arteries there was no difference in MFV and PI at the MCA between axillary and femoral groups. There was a statistically significant trend of bilaterally increased MFV in the axillary group that they attributed to higher flow rates. The study was limited by its sample size and the lack of a control group [39].
Electroencephalography–EEG–Supplemental Table 2
Critically ill patients admitted to ICUs are at an elevated risk of developing seizures [18, 27, 40,41,42,43,44]. Non-convulsive seizures (NCS) in comatose patients can only be diagnosed by EEG. Strikingly, NCS rates can range from 7 to 18% in critically ill patients, with an even higher incidence of epileptiform discharges in up to 22% [45,46,47].
The American Clinical Neurophysiology Society recommended, in a 2015 consensus statement, continuous EEG monitoring for ECMO patients requiring paralysis and who are at risk of seizures. EEG has predictive value using background reactivity for outcomes in comatose patients. Lack of reactivity has been reported as a poor prognostic sign in patients with depressed levels of consciousness [48]. Epileptiform activity in critically ill patients has been associated with poor outcomes [49].
Applications of EEG in pediatric ECMO population
In 1992, Strelets et al. showed that EEG features did not significantly correlate with ECMO cannulation sites. Most abnormal EEG findings were generalized or bilateral and improved following discontinuation of ECMO. EEG changes did not correlate with structural abnormalities on head ultrasound. Seizures were associated with poor outcomes, defined as either death or delay in further development [50].
In 1993, Hahn et al. reported ECMO patients had more repetitive or periodic discharges from the right hemisphere (36 vs 23% in the control group). This was proposed to relate to the right common carotid artery ligation during ECMO cannulation [51]. There were also more right-sided IS in their ECMO group (13.8 vs 0%, p < 0.05). That study did not show any difference in lateralization of electrographic seizures between the ECMO and control groups. Within the ECMO group, the presence of electrographic seizures, status epilepticus and burst suppression patterns were associated with unfavorable outcomes compared to patients with favorable outcomes [51].
In another study evaluating the relation between EEG abnormalities and post-ECMO neuroimaging, 3 of 10 patients suffered from neurologic complications. One patient on V-A ECMO showed a markedly asymmetric EEG background with right hemispheric low amplitude and slow activity correlating with right hemispheric IS seen on neuroimaging post-ECMO. This patient survived with left-sided impairments that were nearly resolved on follow-up. Of note, the patient was treated with antiepileptics and osmotic agents after the abnormal EEG. Another patient suffered a global hypoxic injury and the EEG showed slow diffuse activity. The third patient had bilateral venous infarctions and the EEG showed slow activity in the bilateral posterior head regions with subclinical seizures originating in the right. The latter 2 patients were also treated with antiepileptic drugs and osmotic agents after the EEG abnormalities were detected but suffered severe neurological outcomes on follow-up [52].
Lin et al. studied 99 patients undergoing V-A or V-V ECMO support. None of the V-V ECMO patients experienced electrographic seizures. However, 21% of V-A ECMO patients experienced electrographic seizures. The only statistically significant risk factor that was associated with seizures was low cardiac output. Additionally, although statistically insignificant, seizures did not occur in patients when their initial EEG background was normal or attenuated-featureless [53].
In a study by Sansevere et al. out of 75 patients managed with V-A ECMO, 20 had a bilateral injury; 26 had left hemispheric lesions; and 29 had isolated right hemispheric lesions. It was shown that right-sided lesions were more associated with right common carotid (peripheral) cannulation and left-sided lesions were associated with ascending aortic (central) cannulation (p = 0.03). In this study, electrographic seizures and epileptiform discharges did correlate with the side of injury (p = 0.005). They also found that using antiseizure medications as clinically indicated for a convulsive seizure prior to EEG start shortened the burden of electrographic seizures from 75.3 to 19.3 min (p = 0.04). The higher burden of electrographic seizures in the initial 24 h of EEG monitoring was associated with death and severe background abnormalities (including attenuated/featureless or burst suppression in pediatrics and burst suppression or electrographic inactivity in neonates) and had 96% specificity as a prognosticator for death [54].
Applications of EEG in adult ECMO population
Sinnah et al. showed the presence of a discontinuous or unreactive background on 30-min EEG and lack of sleep architecture on continuous EEG. These were associated with poor outcomes (composite acute brain injury or death at 14 days). There were no differences in the sedation dosing between patients with or without background abnormalities or absence and presence of sleep transients [55]. Cho et al. showed that poor EEG reactivity and poor background variability were associated with poor outcomes (defined as Cerebral Performance Category 3–5). The EEG backgrounds had a fair mix of theta delta or delta frequencies not typically associated with poor outcomes. Most of the 13 patients included did not have a neurological exam indicative of a catastrophic injury to the brain or brainstem and only 2 had neuroimaging findings that can explain being comatose (anoxic injury in one and right thalamic and occipital infarcts with midline shift in the other) [56].
In 2020, Peluso and colleagues reported no difference in EEG findings between their V-V and V-A ECMO groups. Moreover, they found that a severely suppressed background (defined by the absence of any EEG activity at < 10 μV during the entire epoch) and nonreactivity were independently associated with worse outcomes (as measured by GOS at 3 month). However, the presence of seizures (8%) or periodic discharges (7%) did not associate with worse outcomes. Additionally, they showed that a change in the symmetry of the background can be an indicator of underlying neurological insult. In one patient a diffuse low voltage activity was noted on day 5 and developed left hemispheric attenuation secondary to IS on neuroimaging. In addition, in one patient there was the detection of nonconvulsive status epilepticus on EEG which influenced treatment and resulted in a good outcome [57].
On a similar note, Magalhaes and colleagues, in 2020, showed that combining unreactive background with a background slowing to equal or less than 4 Hz had a 0% false positive rate for predicting worse outcomes at 28 (defined by death) and 90 (defined by death or an mRS of 4–6) days, in their V-A ECMO population. However, after adjusting for covariates, a low background frequency was the only independent association with the primary unfavorable outcome at 28 days. This study was limited by the short 30-min EEG monitoring and that only one patient experienced status epilepticus [58].
Recently, Touchard et al. have shown that even a simplified 4 lead frontal EEG can allow the intensivist to pick up common EEG patterns associated with poor outcomes (Death at 28 days or 90-day mRS of 4–6). They used Synek scores (Ranging between 1 as benign and 5 as malignant) as the predictor of poor outcomes based on the score’s correlation with EEG background discontinuity and rhythm [59].
Somatosensory evoked potential—SSEP
SSEP has been used for several years as a prognostic tool for patients with hypoxic-ischemic encephalopathy and an absence of response has been associated with poor neurological outcomes. [60, 61] Cho et al. in 2019, studied 13 ECMO patients who underwent SSEP. The entire cohort had normal or delayed responses in at least one hemisphere. In the 6 patients with a delayed response, only one patient had a bilateral hemisphere delay pattern and NCCT showed a non-correlating right cerebellar infarct. However, in 1 patient an absent left median response correlated with a right thalamic infarct. Twelve patients of these 13 patients suffered a poor neurologic outcome [56]. In 2020 Cho et al. studied a cohort of 20 ECMO patients in which 7 patients had SSEPs with intact N20 responses despite having poor outcomes [34]. This reinforces the need for more data to determine the value of SSEP in neurological monitoring in ECMO patients.
Near-infrared spectroscopy—NIRS
NIRS has been evaluated in ECMO patients for its potential to detect brain injury. A prospective observational study by Hunt et al. assessed the balance between cerebral oxygen delivery and consumption-through regional oxygenation tissue saturation (rSO2), to detect acute brain injury in V-A ECMO. A drop in rSO2 of > 25% below baseline indicated an acute brain injury with a sensitivity of 86% [62]. A retrospective analysis by Pozzebon et al. showed that cerebral desaturation indicating an acute brain injury was detected by NIRS in 74% of patients undergoing V-A ECMO [63].
Neurologic adverse events in ECMO patients
Patients on ECMO are at substantial risk of neurologic adverse events with an incidence ranging from 10.9 to 50%. These include ICH, acute ischemic stroke (AIS), seizures, diffuse cerebral edema, hypoxic brain injury, global and focal hypoperfusion or hyperperfusion injury and brain death [18, 19, 27, 40,41,42,43,44]. Lorusso et al. reviewed the ELSO registry of 4522 patients and identified 15.1% developed neurological complications: brain death (7.9%), AIS (3.6%), and equal incidence of seizures and ICH (1.8%). More than one complication in the same patient occurred in 1.5%. A catastrophic rate of in-hospital mortality reached 100% in patients with 3 or more neurological insults [43]. The reported frequencies of ICH range between 3.6 and 15%; AIS between 4.1 and 9%; and cerebral edema, 13%. Seizures are reported in 4.1% and up to 18% in one study in pediatrics, of which 83% were purely electrographic [18, 41, 53]. Additionally, AIS increases the risk of seizures in ECMO patients [22]. ECMO patients are also at risk of developing cerebral microbleeds [23]. Cerebral hemorrhages in this patient population are also reported to be at a higher risk with a positive family history of intracranial bleeding [64]. Complications during V-A ECMO with intra-aortic balloon pump (IABP) include spinal cord infarctions and peripheral neuropathy, form either a direct injury to the nerve, vascular compromise or compressive injury from a hematoma [65].
Cerebral herniation from ICH or brain edema from traumatic brain injury has been considered by some an absolute contraindication to ECMO which is associated with a need for systemic anticoagulation. There is, however, a lack of evidence for nonfatal ICH serving as a contraindication to ECMO cannulation [66]. More recent studies show that consideration of an anti-coagulation-free regimen may be a safe and feasible option with similar rates of thrombotic events and bleeding of systemic anticoagulation in certain ECMO patients [67,68,69].
Luyt et al. conducted an observational study and a systematic review that included 135 patients requiring V-V ECMO support. This study identified 18 patients with cerebral complications while on ECMO, including cerebral bleeding (7.5% n = 10), ischemic stroke (2%, n = 2) and diffuse microbleeds (2%, n = 2) [15]. Another case series by Martucci et al. demonstrated 6 cases who suffered from cerebral complications while on V-V ECMO. This included ischemic stroke due to fat embolism in 1 case, intracranial hemorrhage in 4 cases, and acute hemorrhagic encephalomyelitis in 1 case [70]. In a retrospective analysis of 878 patients undergoing V-A ECMO, 65 (7.4%) developed a cerebral insult, which included ischemic stroke (5.3%) and intracranial bleeding (2.8%) [16].
Discussion
ECMO is a lifesaving intervention in adults and pediatric patients for the support of severe cardiac and pulmonary dysfunction refractory to medical management [71]. Unfortunately, patients being treated with ECMO are at a higher risk for neurological complications that are disabling or acutely fatal [72]. The causal relationships are less clear and vary between adult and pediatric populations as well as the ECMO configuration (V-A vs. V-V). Identifying neurological disturbances and complications remain time sensitive, is challenging and may be overlooked.
This narrative literature review focused on understanding the landscape of neuromonitoring modalities as it applies to patients receiving ECMO support. We have described challenges posed by traditional evaluation methods for identifying neurological disturbances such as the physical examination or obtaining neuromonitoring modalities that can be utilized at the bedside remain under investigation for ECMO patients. Research is growing in EEG monitoring, TCDs with microemboli detection, and SSEPs in predicting complications. There remains a lack of consensus or guidance in how to interpret the results for identifying neurological insults in inpatients on ECMO. TCDs can predict neurological complications by quantifying blood flow velocity and MES detection, however, these require experienced personnel in acquisition and interpretation. EEG is a helpful bedside modality and identification of epileptiform activity may have its greatest role in neurological prognostication for patients on ECMO. SSEPs are helpful in localization, but most recent literature suggests a limited understanding of their utility in prognosis and further studies are needed on its use. From a practical perspective, the general day-to-day management of patients on ECMO does not emphasize a need to consider neurological disturbances. Kazmi et al. describe a neuro-surveillance protocol that incorporates NCCT, TCD, EEG and NIRS in combination with goal-directed anticoagulation monitoring and a multidisciplinary team-based approach for neurological monitoring in ECMO patients [73]. Understanding the challenges and limitations of neurological assessments in ECMO patients can inform the development of local protocols that focus on neuro-surveillance in this growing patient population.
Conclusion
Neurological monitoring is an important area of focus to identify disturbances associated with the initiation of ECMO support and as a complication of continued ECMO support. The available literature is limited, heterogenous and lacks consensus on the best practices for neurological monitoring. Future studies should focus on the optimal measures of neurological monitoring modalities and utilization in the ECMO patient that can inform bedside evaluation and management.
Data availability
Data availability statement is not applicable for this study type.
Abbreviations
- ECMO:
-
Extracorporeal membrane oxygenation
- ECLS:
-
Extracorporeal life support
- ICH:
-
Intracerebral hemorrhage
- MRI:
-
Magnetic resonance imaging
- NCCT:
-
Non-contrast computed tomography
- CTA:
-
CT angiography
- CTP:
-
CT perfusion
- CBF:
-
Cerebral blood flow
- CCA:
-
Conventional cerebral angiogram
- CMB:
-
Chronic microbleed
- MES:
-
Microembolic signals
- TCD:
-
Transcranial doppler
- MFV:
-
Mean flow velocity
- EEG:
-
Electroencephalography
- SSEP:
-
Somatosensory evoked potential
References
Bartlett RH (2014) John H Gibbon Jr Lecture. Extracorporeal life support: Gibbon fulfilled. J Am Coll Surg 218(3):317–327
Chavez P, Messerli FH, Casso Dominguez A, Aziz EF, Sichrovsky T, Garcia D et al (2015) Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Hear 2(1):e000257
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ et al (2004) Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 240(4):595–597
Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK (1997) Extracorporeal membrane oxygenation for adult respiratory failure. Chest 112(3):759–764
Lewandowski K, Rossaint R, Pappert D, Gerlach H, Slama KJ, Weidemann H et al (1997) High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med 23(8):819–835
Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH (1997) Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg 226(4):544–546
Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A et al (2013) Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med 39(2):275–281
Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C et al (2006) CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 6(1):163. https://doi.org/10.1186/1472-6963-6-163
Sameed M, Meng Z, Marciniak ET (2019) EOLIA trial: the future of extracorporeal membrane oxygenation in acute respiratory distress syndrome therapy? Breathe (Sheff) 15(3):244–246
Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD et al (2017) Extracorporeal life support organization registry international report 2016. ASAIO J 63(1):60–67
Ortega-Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E (2016) Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: a systematic review of international practices and outcomes. Resuscitation 101:12–20
Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA et al (2020) Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet (London, England) 396(10265):1807–1816
de St Maurice AM, Bridges BC, Rycus PT, Fonnesbeck CJ, Fleming GM, Halasa NB (2016) Global trends in extracorporeal membranous oxygenation use and survival of patients with influenza-associated illness. Pediatr Crit Care Med 17(9):876–883
Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C (2014) Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 18(1):203
Luyt C-E, Bréchot N, Demondion P, Jovanovic T, Hékimian G, Lebreton G et al (2016) Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med 42(5):897–907
Le Guennec L, Cholet C, Huang F, Schmidt M, Bréchot N, Hékimian G et al (2018) Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care 8(1):129
Extracorporeal Life Support Organization. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support , Version 1.4. 2017; Available from: www.elso.org
Lidegran MK, Mosskin M, Ringertz HG, Frenckner BP, Lindén VB (2007) Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol 14(1):62–71
Aboul Nour H, Poyiadji N, Mohamed G, Alsrouji OK, Ramadan AR, Griffith B et al (2020) Challenges of acute phase neuroimaging in VA-ECMO, pitfalls and alternative imaging options. Interv Neuroradiol 27:434–439
Acharya J, Rajamohan AG, Skalski MR, Law M, Kim P, Gibbs W (2017) CT Angiography of the head in extracorporeal membrane oxygenation. Am J Neuroradiol 38(4):773–776
Jepson SL, Harvey C, Entwisle JJ, Peek GJ (2010) Management benefits and safety of computed tomography in patients undergoing extracorporeal membrane oxygenation therapy: experience of a single centre. Clin Radiol 65(11):881–886
LaRovere KL, Vonberg FW, Prabhu SP, Kapur K, Harini C, Garcia-Jacques R et al (2017) Patterns of head computed tomography abnormalities during pediatric extracorporeal membrane oxygenation and association with outcomes. Pediatr Neurol 73:64–70
Le GL, Bertrand A, Laurent C, Roze H, Chastre J, Combes A et al (2015) Diffuse cerebral microbleeds after extracorporeal membrane oxygenation support. Am J Respir Crit Care Med 191(5):594–596
Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y et al (2017) Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds. Neurology 89(8):820–829
Liebeskind DS, Sanossian N, Sapo ML, Saver JL (2013) Cerebral microbleeds after use of extracorporeal membrane oxygenation in children. J Neuroimaging 23(1):75–78
Cho S-M, Wilcox C, Keller S, Acton M, Rando H, Etchill E et al (2022) Assessing the SAfety and FEasibility of bedside portable low-field brain magnetic resonance imaging in patients on ECMO (SAFE-MRI ECMO study): study protocol and first case series experience. Crit Care 26(1):119
Xie A, Lo P, Yan TD, Forrest P (2017) Neurologic complications of extracorporeal membrane oxygenation: a review. J Cardiothorac Vasc Anesth 31(5):1836–1846
Kavi T, Esch M, Rinsky B, Rosengart A, Lahiri S, Lyden PD (2016) Transcranial doppler changes in patients treated with extracorporeal membrane oxygenation. J Stroke Cerebrovasc Dis 25(12):2882–2885
Rilinger JF, Smith CM, deRegnier RAO, Goldstein JL, Mills MG, Reynolds M et al (2017) Transcranial doppler identification of neurologic injury during pediatric extracorporeal membrane oxygenation therapy. J Stroke Cerebrovasc Dis 26(10):2336–2345
O’Brien NF, Buttram SDW, Maa T, Lovett ME, Reuter-Rice K, LaRovere KL et al (2019) Cerebrovascular physiology during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 20(2):178–186
O’Brien NF, Hall MW (2013) Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med 14(3):e126–e134
Zanatta P, Forti A, Bosco E, Salvador L, Borsato M, Baldanzi F et al (2010) Microembolic signals and strategy to prevent gas embolism during extracorporeal membrane oxygenation. J Cardiothorac Surg 5(1):5
Marinoni M, Migliaccio ML, Trapani S, Bonizzoli M, Gucci L, Cianchi G et al (2016) Cerebral microemboli detected by transcranial doppler in patients treated with extracorporeal membrane oxygenation. Acta Anaesthesiol Scand 60(7):934–944
Cho S-M, Ziai W, Mayasi Y, Gusdon AM, Creed J, Sharrock M et al (2020) Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J 66(4):388–393
Chung M, Shiloh AL, Carlese A (2014) Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. Sci World J 2014:1–10
Marinoni M, Cianchi G, Trapani S, Migliaccio ML, Bonizzoli M, Gucci L et al (2018) Retrospective analysis of transcranial doppler patterns in veno-arterial extracorporeal membrane oxygenation patients. ASAIO J 64(2):175–182
Bembea MM, Felling R, Anton B, Salorio CF, Johnston MV (2015) Neuromonitoring during extracorporeal membrane oxygenation. Pediatr Crit Care Med 16(6):558–564
Yang F, Jia Z, Xing J, Wang Z, Liu Y, Hao X et al (2014) Effects of intra-aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med 12(1):106
Salna M, Ikegami H, Willey JZ, Garan AR, Cevasco M, Chan C et al (2019) Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J Card Surg 34(6):447–452
Bowling SM, Gomes J, Firstenberg MS (2016) Neurologic issues in patients receiving extracorporeal membrane oxygenation support. In: Firstenberg MS (ed) Extracorporeal membrane oxygenation-advances in therapy. IntechOpen, pp 321–337. https://doi.org/10.5772/64269
Nasr DM, Rabinstein AA (2015) Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol 11(4):383–389
Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EFM (2011) Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol 68(12):1543–1549
Lorusso R, Barili F, Di MM, Gelsomino S, Parise O, Rycus PT et al (2016) In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation. Crit Care Med 44(10):e964–e972
Risnes I, Wagner K, Nome T, Sundet K, Jensen J, Hynås IA et al (2006) Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg 81(4):1401–1406
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ (2004) Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 62(10):1743–1748
Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR (2004) Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol 61(7):1090–1094
Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ (2009) Continuous electroencephalography in the medical intensive care unit&ast. Crit Care Med 37(6):2051–2056
Azabou E, Navarro V, Kubis N, Gavaret M, Heming N, Cariou A et al (2018) Value and mechanisms of EEG reactivity in the prognosis of patients with impaired consciousness: a systematic review. Crit Care 22(1):184
Zafar SF, Rosenthal ES, Jing J, Ge W, Tabaeizadeh M, Aboul Nour H et al (2021) Automated annotation of epileptiform burden and its association with outcomes. Ann Neurol 90(2):300–311
Streletz LJ, Bej MD, Graziani LJ, Desai HJ, Beacham SG, Cullen J et al (1992) Utility of serial EEGs in neonates during extracorporeal membrane oxygenation. Pediatr Neurol 8(3):190–196
Hahn J, Vaucher Y, Bejar R, Coen R (1993) Electroencephalographic and neuroimaging findings in neonates undergoing extracorporeal membrane oxygenation. Neuropediatrics 24(01):19–24
Jeong IS, Woo YJ, Kim DW, Kim NY, Cho HJ, Ma JS (2014) Efficacy of electroencephalographic monitoring for the evaluation of intracranial injury during extracorporeal membrane oxygenation support in neonates and infants. Korean J Crit Care Med 29(2):70–76
Lin N, Flibotte J, Licht DJ (2018) Neuromonitoring in the neonatal ECMO patient. Semin Perinatol 42(2):111–121
Sansevere AJ, DiBacco ML, Akhondi-Asl A, LaRovere K, Loddenkemper T, Rivkin MJ et al (2020) EEG features of brain injury during extracorporeal membrane oxygenation in children. Neurology 95(10):e1372–e1380
Sinnah F, Dalloz M-A, Magalhaes E, Wanono R, Neuville M, Smonig R et al (2018) Early electroencephalography findings in cardiogenic shock patients treated by venoarterial extracorporeal membrane oxygenation. Crit Care Med 46(5):e389–e394
Cho S-M, Choi CW, Whitman G, Suarez JI, Martinez NC, Geocadin RG et al (2019) Neurophysiological findings and brain injury pattern in patients on ECMO. Clin EEG Neurosci 52:462–469
Peluso L, Rechichi S, Franchi F, Pozzebon S, Scolletta S, Brasseur A et al (2020) Electroencephalographic features in patients undergoing extracorporeal membrane oxygenation. Crit Care 24(1):629
Magalhaes E, Reuter J, Wanono R, Bouadma L, Jaquet P, Tanaka S et al (2020) Early EEG for prognostication under venoarterial extracorporeal membrane oxygenation. Neurocrit Care 33(3):688–694
Touchard C, Cartailler J, Vellieux G, de Montmollin E, Jaquet P, Wanono R et al (2021) Simplified frontal EEG in adults under veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care 11(1):76
Zandbergen EGJ, de Haan RJ, Stoutenbeek CP, Koelman JHTM, Hijdra A (1998) Systematic review of early prediction of poor outcome in anoxicischaemic coma. Lancet 352(9143):1808–1812
Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL (2003) Predictive value of somatosensory evoked potentials for awakening from coma&ast. Crit Care Med 31(3):960–967
Hunt MF, Clark KT, Whitman G, Choi CW, Geocadin RG, Cho S-M (2021) The use of cerebral NIRS monitoring to identify acute brain injury in patients with VA-ECMO. J Intensive Care Med 36(12):1403–1409
Pozzebon S, Blandino Ortiz A, Franchi F, Cristallini S, Belliato M, Lheureux O et al (2018) Cerebral near-infrared spectroscopy in adult patients undergoing veno-arterial extracorporeal membrane oxygenation. Neurocrit Care 29(1):94–104. https://doi.org/10.1007/s12028-018-0512-1
Martucci G, Lo Re V, Marrone G, Caruso S, Arcadipane A (2015) V LoRe (2015) Intracranial hemorrhage during extracorporeal membrane oxygenation: does family history play a role? Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 36:1523–1525
Samadi B, Nguyen D, Rudham S, Barnett Y (2016) Spinal cord infarct during concomitant circulatory support with intra-aortic balloon pump and veno-arterial extracorporeal membrane oxygenation. Crit Care Med 44(2):e101–e105
Harnisch L-O, Moerer O (2021) Contraindications to the initiation of veno-venous ecmo for severe acute respiratory failure in adults: a systematic review and practical approach based on the current literature. Membranes 11(8):584
Kato C, Oakes M, Kim M, Desai A, Olson SR, Raghunathan V et al (2021) Anticoagulation strategies in extracorporeal circulatory devices in adult populations. Eur J Haematol 106(1):19–31. https://doi.org/10.1111/ejh.13520
Olson SR, Murphree CR, Zonies D, Meyer AD, Mccarty OJT, Deloughery TG et al (2021) Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J 67(3):290–296
Murphree CR, Shatzel JJ, Olson SR (2019) Bleeding and thrombotic outcomes in anticoagulant free extracorporeal membrane oxygenation (ECMO) in adults: a systematic review. Blood 134:2436
Martucci G, Lo Re V, Arcadipane A (2016) Neurological injuries and extracorporeal membrane oxygenation: the challenge of the new ECMO era. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 37(7):1133–1136
Shekar K, Mullany DV, Thomson B, Ziegenfuss M, Platts DG, Fraser JF (2014) Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 18(3):219
Mehta A, Ibsen LM (2013) Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit care Med 2(4):40–47
Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C (2018) Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract 2018:1–11
Acknowledgements
The authors would like to thank Dr. Panayiotis N. Varelas, MD, PhD, FAAN, FNCS, for his help with reviewing and editing the manuscript.
Author information
Authors and Affiliations
Contributions
HAN created the idea for the project. HAN, AJ, HA, AS, HB, NJ, JGG contributed to the methodology, literature review and synthesis of the review. HB, NJ, NGG supervised the project. All authors contributed to drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aboul-Nour, H., Jumah, A., Abdulla, H. et al. Neurological monitoring in ECMO patients: current state of practice, challenges and lessons. Acta Neurol Belg 123, 341–350 (2023). https://doi.org/10.1007/s13760-023-02193-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02193-2