Abstract
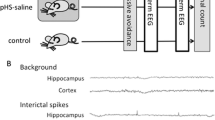
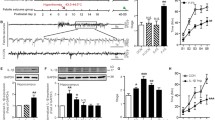
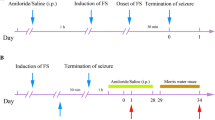
Febrile seizures (FS) are recognized as an antecedent to the development of temporal lobe epilepsy with hippocampal sclerosis (TLE-HS), but it is unclear whether prolonged FS are a direct cause of TLE-HS. Here, we used a rat model of infantile FS to study the effects of inflammatory cytokines on seizure susceptibility and neuronal death in adults. Prolonged hyperthermia-induced seizures (pHS) were induced in male Lewis rats at post natal day (P) 10. Cytokines were administered twice intranasally, once immediately after pHS and once the following day. The effects of intranasal interleukin (IL)-1β or tumor necrosis factor (TNF) α were tested in rats undergoing a single episode of pHS (P10) and in rats undergoing repeated pHS (P10 and P12). Seizure susceptibility was tested at P70–73 by quantifying the seizure onset time (SOT) after kainic acid administration, and neuronal cell injury and gliosis in adulthood. SOT significantly reduced in rats receiving IL-1β together with repeated pHS, whereas no significant effects were seen in rats receiving IL-1β after a single pHS episode, or in rats receiving TNFα. Hippocampal neuronal cell loss was observed in the CA3 region of rats receiving IL-1β together with repeated pHS; however, there was no significant change in gliosis among each group. Our results are consistent with the hypothesis that excessive production of IL-1β after repeated prolonged FS can enhance adult seizure susceptibility and neuronal cell death, and might contribute to the development of TLE-HS.




Similar content being viewed by others
References
Tanabe T, Hara K, Shimakawa S, Fukui M, Tamai H et al (2011) Hippocampal damage after prolonged febrile seizure: one case in a consecutive prospective series. Epilepsia 52:837–840
Dubé C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ (2000) Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol 47:336–344
Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ et al (2006) Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain 129:911–922
Kira K, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, Sakamoto K, Matsumoto S, Gondo K, Hara T et al (2005) Genetic susceptibility to simple febrile seizures: interleukin-1β promoter polymorphisms are associated with sporadic cases. Neurosci Lett 384:239–244
Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ et al (2005) Interleukin-1β contributes to the generation of experimental febrile seizures. Ann Neurol 57:152–155
Fukuda M, Suzuki Y, Ishizaki Y, Kira R, Kikuchi C, Watanabe S, Hino H, Morimoto T, Hara T, Ishii E et al (2009) Interleukin-1β enhances susceptibility to hyperthermia-induced seizures in developing rats. Seizure 18:211–214
Dubé C, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AM, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ et al (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494
Auvin S, Shin D, Mazarati A, Sankar R et al (2010) Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia 51(Suppl 3):34–38
Riazi K, Galic MA, Pittman QJ (2010) Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res 89:34–42
Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, Nishimura M et al (2003) Increased frequency of interleukin-1β-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 44:796–799
Hino H, Takahashi H, Suzuki Y, Tanaka J, Ishii E, Fukuda M et al (2012) Anticonvulsive effect of paeoniflorin on experimental febrile seizures in immature rats: possible application for febrile seizures in children. PLoS ONE 7:e42920
Lawrence D (2002) Intranasal delivery could be used to administer drugs directly to the brain. Lancet 359:1674
Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ et al (2008) Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 28:6904–6913
Gerfen CR (2003) Basic neuroanatomical methods. Curr Prot Neurosci Unit 1.1., pp 1–11
de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD et al (2003) A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia 44:677–687
Bae EK, Jung KH, Chu K, Lee ST, Kim JH, Park KI, Kim M, Chung CK, Lee SK, Roh JK et al (2010) Neuropathologic and clinical features of human medial temporal lobe epilepsy. J Clin Neurol 6:73–80
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW et al (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Zheng XY, Zhang HL, Luo Q, Zhu J et al (2011) Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol 2011:457079
Pitkanen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1:173–181
Yang T, Zhou D, Stefan H (2010) Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: uncontrolled inflammation drives disease progression? J Neurol Sci 296:1–6
Blümcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstöck J, Stefan H, Hildebrandt M et al (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113:235–244
Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S et al (1998) Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology 50:407–411
Auvin S, Shin D, Mazarati A, Nakagawa J, Miyamoto J, Sankar R et al (2007) Inflammation exacerbates seizure-induced injury in the immature brain. Epilepsia 48(Suppl 5):27–34
Auvin S, Mazarati A, Shin D, Sankar R et al (2010) Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis 40:303–310
Lee SH, Kim BJ, Kim YB, Chung PW, Moon HS, Suh BC, Yoon WT, Jin DK, Park YS, Lee YT, Park KY et al (2012) IL-1β induction and IL-6 suppression are associated with aggravated neuronal damage in a lipopolysaccharide-pretreated kainic acid-induced rat pup seizure model. NeuroImmunoModulation 19:319–325
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22:797–803
Ravizza T, Balosso S, Vezzani A (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497:223–230
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartifai T, Binaglia M, Corsini E, Luca MD, Galli CL, Marinovich M et al (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 24:8692–8700
Ravizza T, Noé F, Zardoni D, Vaghi V, Sifringer M, Vezzani A et al (2008) Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol Dis 31:327–333
Johnson D, Amaral DG (2003) Hippocampus. In: Johnson D, Amaral DG (eds) The synaptic organization of the brain, 5th edn. Oxford University Press, Oxford, pp 455–493
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Zastrow MV, Beattie MS, Malenka RC et al (2002) Control of synaptic strength by glial TNFα. Science 295:2282–2285
Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC et al (2004) TNFα-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol 1:263–273
Morimoto T, Kida K, Nagao H, Yoshida K, Fukuda M, Takahashi S et al (1995) The pathogenic role of the NMDA receptor in hyperthermia-induced seizures in developing rats. Dev Brain Res 84:204–207
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology (No.22591132), a Research Grant from the Japan Epilepsy Research Foundation, and a Research Promotion Award from Ehime University. We are grateful to the staff of the Animal Center of INCS of Ehime University for their gentle care of our animals, and Takeshi Kiyoi for help with histological staining.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Hino, H., Suzuki, Y. et al. Postnatal interleukin-1β enhances adulthood seizure susceptibility and neuronal cell death after prolonged experimental febrile seizures in infantile rats. Acta Neurol Belg 114, 179–185 (2014). https://doi.org/10.1007/s13760-013-0246-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-013-0246-y