Abstract
Through the Kabachnic-Fields three-component reaction of 3(2-amino-acetyl)-quinazolin-4(3H)-one, compound III, various aromatic aldehydes, triphenyl phosphite, and lithium perchlorate as Lewis acid catalyst, new α-amino phosphonates molecules, IVa-f have been produced in a high yield. FT-IR, 1H-NMR, elemental analysis, and mass spectral data were used to determine the structures of the newly synthesized chemicals. Examined phosphonates, Iva–f, have been tested for their in vitro anticancer effects on the five cell lines HePG-2, MCF-7, Hela, HCT-116, PC-3, and normal cell, WI-38. Newly synthesized compounds' antioxidant activities were also covered. The novel-created α-amino phosphonate compounds have been evaluated on six cell lines and exhibit good anti-proliferative properties. The IVc molecule is the most effective antioxidant and anticancer candidate. Utilizing DFT/B3LYP/6-311G (d, p) method, the electronic and geometric characteristics derived from the stable structure of the studied compounds were examined. Additionally, there are outcomes for HOMO–LUMO, molecule electrostatic potential, and quantum chemical parameters. The stability of the most active phosphonate molecule, IVc, is attributed to hyper-conjugative interactions and charge delocalization. This was investigated using NBO analysis. Theoretical FT-IR and 1H-NMR measurements were applied to demonstrate the relationship between theory and experiment. An excellent concurrence between experimental and theoretical data was discovered. A docking simulation study was applied to forecast the inhibitory mode of action of the most active substance inside the cavity of estrogen receptor-positive (ER +) MCF-7 breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic chemistry contains at least half of all organic chemistry studies globally and is likewise referred to as the largest of classical organic synthesis [1, 2]. More than 90% of new drugs include heterocyclic, which performs a crucial function in processes and industries as an interface between chemistry and biology [3]. Among all heterocyclic compounds, quinazolines are N-containing fused heterocyclic compounds with great utility in biological applications [4]. The quinazolin-4(3H)-one and its derivatives represent a vital class of fused heterocycles that possess a broad spectrum of biological activity, such as anticancer [5, 6], antitumor [7,8,9,10,11], antioxidant [12], antimicrobial [13, 14], antifungal [15], anticonvulsant [16, 17], anti-HIV [18], anti-HCV [19] and anti-TMV [20] activities. The pharmacological properties and clinical applications of α-amino phosphonic acids and their corresponding α-amino phosphonates have been widely studied [21,22,23]. Their potential biological effects as potent enzyme inhibitors, antibacterial, anticancer, antioxidant, and antiviral drugs drew much attention [24,25,26,27,28,29]. The aminophosphonate group in the pharmacy core has been shown to increase anticancer action. Furthermore, many amino phosphonates have shown substantial inhibitory properties against human malignancies [30, 31]. Quinazolinone derivatives and amino phosphonates have distinct biological and pharmaceutical properties. Therefore, structural changes of quinazolinone derivatives by inserting the α-aminophosphonate group boost pharmacological potency. [32,33,34,35]. In recent decades, advances in computational chemistry have partnered with experience in studying biological and biochemical structures and reactions to create a complete partner [36]. It has contributed significantly to understanding structure, molecular characteristics, processes, and reaction selectivity [36]. It also lets us comprehend the compound's likely behavior during reactions and provides crucial information about the compounds under investigation. These include total energy, binding energy, electronic energy, dipole moment, bond lengths, HOMO, and LUMO, and when this knowledge agrees with experimental evidence, its high value grows [37]. The applicability of semi-empirical methods/PM6 for calculating newly synthesized chemicals has been assessed [38, 39]. Also, DFT theory is an important method used in calculating the properties of molecules [40,41,42,43]. The present work aims to synthesize new quinazoline compounds bearing phosphonate group by studying anticancer in vitro and antioxidant activity. In addition, the influence of changes in molecular and electronic structures on the biological activity of newly synthesized molecules was investigated using semi-empirical and DFT approaches. Also, see if we can find a link between the experimental and theoretical results.
Experimental
The compound melting points in degree centigrade through the capillary technique of Gallenkamp without correction have been suggested. The FT-IR spectra (KBr) on a Mattson 5000 FT-IR spectrophotometer, using a significant laboratory at TantaUniv, had been reported. Elemental analyses (C, H, and N) were accomplished at the Microanalytical Center of CairoUniv., Egypt. The 1H-NMR spectra have been achieved at Kafr El-sheikhUniv., Egypt, on a BucherAC400 spectrometer working at four hundred MHz. The spectra have been recorded in DMSO-d6, and chemical shifts δ have been suggested relative to TMS. The MS had been recorded on Shimadzu Qp-2010 plus at the Microanalytical Center, CairoUniv. All samples were tested using analytical (TLC), which changed into accomplished on EM silica-gel F254 sheet, 0.2 mm. The biological evaluation was brought to the pharmacognosis department, Mans. Univ., Egypt.
Synthesis 3-(2-amino-acetyl)-quinazolin-4(3H)-one, III
4(3H)-quinazolinone, II [44],0.01 mol, and glycine ethyl ester, 0.01 mol, in pyridine (freshly distilled and dried) were refluxed in a round bottom flask for 12 h.
Finally, excess pyridine is distilled out beneath decreased pressure; then, the solution is poured directly into a beaker containing beaten ice. It was filtered below suction, rinsed with ice-cold water in parts, and dried at 60 °C. The product was refined using ethanol recrystallization to produce a white crystalline solid of 3-(2-amino-acetyl)-quinazoline-4 (3H)-one, III, in an excellent yield (Fig. 1).
The molecular formula is C10H9N3O2, molecular weight is 203, yield is 78%, and m.p is 188–190 °C.
The compound structure III has been confirmed by:
-
Correct analytical data for C10H9N3O2 (230): Calculated: C, 59.11; H, 4.46; N, 20.68%, Found: C, 59.34; H, 4.63; N, 20.92%.
-
FT-IR (KBr): 3439, 3127, 2974, 1705, 1667, 1613, 1563, 1468 cm-1.
-
1H-NMR: δ 2.52 (s, 2H, CH2), 9.16 (s, 2H, NH2 aliphatic amino), 7.22–8.77 (m, 5H, quinazolineprotons).
-
MS spectra show the peak of molecular ions (M +) on M/Z = 203 (1.35), 171 (1.19), 146 (35.40), 119 (40.86), 91 (64.73), 77 (18.05), 57 (100).
General procedure for the synthesis of diphenyl [(aryl) ((2-oxo-2-(4-oxo-quinazoline-3(4H)-yl) ethyl)amino) methyl] phosphonate, IVa-f
Different aldehydes solution (0.012 mol) in dry dichloromethane 5 mL, triphenyl phosphite,0.01 mol, and lithium perchlorate, LiClO4, (10 mol percent) were added to a solution of 3-(2-amino-acetyl)-quinazoline-4 (3H)-one, III, 0.01 mol in dry dichloromethane 5 mL. At room temperature, the reaction mixture is agitated until the reaction settles, as determined by TLC (48–70 h). Then, α-aminophosphonate compounds, IVa–f, yielded very good after evaporating CH2Cl2 and recrystallization from ethanol (Fig. 2).
Diphenyl[(4'-nitrophenyl)((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVa: 48 h, yield: 86%, m.p. 132–134 °C
The shape of compound IVa turned into shown by:
-
Correct analytical records for C29H23N4O7P (570.49): Calculated: C, 61.05; H, 4.06; N, 9.82%, Found: C, 61.21; H, 4.32; N, 9.97%.
-
IR: 3420, 3268, 3222, 3185, 3070, 2912, 1710, 1668, 1651, 1642, 1633, 1595, 1517, 1488, 1341, 1210, 1063, 1017, 771 cm-1.
-
1H-NMR: δ 2.50 (s, 2HCH2), 5.70–5.74 (d, 1H, CH–P), 8.57 (s, 1H, NH), 6.772–7.603 (m,10H and2 Ph-O(1)), 7.74–8.126 (m,4H andAr-H (2)),8.169–8.295 (m,5H, protons quinazoline (3)).
-
Spectra MS shows the peak of molecular ions (M +) at m/z = 570 (4.1), 538 (4.8), 384.60 (9.7), 297 (4.7), 217 (3.3), 118 (25.88), 91.10 (95.73), 57 (100).
Diphenyl[(4'-hydroxyphenyl)((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVb: 50 h, 80% yield, m.p. 202–204° C
IVb compound structure was confirmed
-
Correct analytical for C29H24N3O6P (541.49): Calc.: C, 64.32; H, 4.47;N, 7.76%, Found: C, 64.47; H, 4.58; and N, 7.92%.
-
IR: 3377, 3254, 3063, 3013, 2917, 2852, 1711, 1665, 1651, 1630, 1610, 1579, 1560, 1519, 1438, 1350, 1236, 1069, 974, 763 cm-1.
-
1H-NMR: δ 2.50 (s, 2H,CH2), 4.95–5.01 (d, 1HCH-P), 8.66(s,1H, and NH), 9.80 (s, 1H, OH), 5.96–7.43 (m,10H and 2Ph-O (1)),7.62–7.92(m,4H, and Ar–H (2))8.17–8.39 (m,5H, protons quinazoline(3)).
-
MS spectra confirmed the peak of molecular ion(M +)atm/z = 541.49 (5), 498 (17), 307 (17), 297 (11), 146 (100).
Diphenyl[(2',4'-dihydroxyphenyl)((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVc: 58 h, yield: 85%, m.p. 196–198 °C
IVc compound structure is confirmed
-
Correct analytical data for C29H24N3O7P (557.49): Calculated: C, 62.48; H, 4.34; N, 7.54%, Found: C, 62.58; H, 4.52; N, 7.66%.
-
FT-IR (KBr): 3370, 3228, 3178, 3076, 2955, 2915, 1712, 1662, 1641, 1631, 1611, 1590, 1560, 1510, 1479, 1357, 1246, 1065, 1014, 772 cm-1.
-
1H-NMR (DMSO): δ 2.52 (s, 2H, CH2), 5.24–5.26 (d, 1H, CH–P), 7.92 (s, 1H, NH), 8.20 (s, 1H, OH), 8.58 (s, 1H, OH) 5.55–6.68 (m,10H, and 2 Ph-O(1)), 6.87–7.19 (m,3H,Ar–H (2)),7.19–7.76 (m,5H, protons quinazoline (3)).
-
MS spectra confirmed the peak of molecular ion (M +) atm/z = 557(4), 423 (12.5), 313.25 (9.51), 269 (13.5), 146 (100), 76 (16.67), 64.05 (40.34)
Diphenyl [(3', 4'-dimethoxyphenyl) ((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVd: 52 h, yield: 91%, m.p. 222–224° C.
The structure of compound IVd was confirmed by:
-
Correct analytical data for C31H28N3O7P (585.54): Calculated: C, 63.59; H, 4.82; N, 7.18%, Found: C, 63.65; H, 4.96; N, 7.32%.
-
FT-IR (KBr): 3420, 3227, 3176, 3065, 3016, 2914, 1703, 1662, 1642, 1622, 1612, 1591, 1553, 1509, 1479, 1357, 1249, 1065, 1018, 767 cm-1.
-
1H-NMR (DMSO): δ 2.52 (s, 2H, CH2), 3.70, 3.74 (s, 6H, 2CH3), 5.02–5.08 (d, 1H, CH–P), 8.65 (s, 1H, NH), 6.69–7.65 (m, 10H, 2 Ph–O (1)), 7.73–7.90 (m, 3H, Ar–H (2)), 7.91–8.17 (m, 5H, quinazoline protons (3)).
Diphenyl [(4'-bromophenyl) ((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVe: 60 h, yield: 93%, m.p. 148–150° C.
The structure of compound IVe was confirmed by
-
Correct analytical data for C29H23 BrN3O5P (604.39): calculated: C, 57.63; H, 3.84; N, 6.95%, Found: C, 57.76; H, 3.94; N, 7.01%.
-
FT-IR (KBr): 3420, 3228, 3177, 3076, 2954, 2914, 1712, 1661, 1641, 1621, 1611, 1560, 1540, 1519, 1471, 1355, 1252, 1068, 1017, 766 cm-1.
-
1H-NMR (DMSO): δ 2.51 (s, 2H, CH2), 5.48–5.45 (d, 1H, CH–P), 8.61 (s, 1H, NH), 5.95–7.63 (m, 10H, 2 Ph–O (1)), 7.73–7.89 (m, 4H, Ar–H (2)), 7.93–8.19 (m, 5H, quinazoline protons (3)).
Diphenyl [(4'hydroxy, 3'-methoxyphenyl) ((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate IVf: 57 h, yield: 89%, m.p. 228–230 °C.
The structure of compound IVf was confirmed by
-
Correct analytical data for C30H26N3O7P (571.52): Calculated: C, 63.05; H, 4.59; N, 7.35%, Found: C, 63.19; H, 4.73; N, 7.42%.
-
FT-IR (KBr): cm-1.
-
1H-NMR (DMSO): δ 2.53 (s, 2H, CH2), 3.98 (s, 3H, CH3), 5.95–5.98 (d, 1H, CH–P), 8.84 (s, 1H, NH), 9.79 (s, 1H, OH), 6.16–7.174 (m, 10H, 2 Ph–O (1)), 7.37–7.57 (m, 3H, Ar–H (2)), 7.71–8.18 (m, 5H, quinazoline protons (3)).
Antitumor activity
The cytotoxic activity of the compounds under investigation against HepG2, MCF-7, Hela, HCT-116, PC-3, and WI-38 was briefly described utilizing doxorubicin as a reference anticancer medicinal medication (supplementary data).
Antioxidant assay
The bleaching of ABTS-produced radical cations and ascorbic acid as a standard antioxidant (positive control) were used to determine the antioxidant interest of the synthesized compounds (supplementary data).
Theory of calculations
The Gaussian 09W program packages created by Frisch and coworkers were used to execute all quantum chemistry calculations on a home computer [45]. The structure of the molecules is optimized using the semi-empirical/PM6 approach, then DFT with Beck's three-parameter exchange functional and nonlocal correlation functional, Lee–Yang–Parr, B3LYP [46,47,48] utilizing the 6-311G(d,p)basis set. Gaussian view 05 software)[49] was used to visualize the charge density distribution of the HOMO (Highest occupied molecular orbital), LUMO (Lowest unoccupied molecular orbital), and MEP (Molecular electrostatic potential). The consensus docking was carried out employing Molegro Virtual Docker (MVD) program [50].
Results and discussion
Chemistry
The refluxing anthranilic acid, I, with formamide gives 4(3H)-quinazolinone, II, which on treatment with glycine ethyl ester in pyridine under refluxing gives 3(2-amino-acetyl)-quinazolin-4(3H)-one, III, Scheme 1.
In the presence of lithium perchlorate that acts as a Lewis acid catalyst, the reaction of 3(2-amino-acetyl)-quinazolin-4(3H)-one,III, with various aromatic aldehydes and triphenyl phosphite yields novel α-amino phosphonates, IVa–f, Scheme 2.
Corrected elemental analysis (Experimental portion), FT-IR, 1H NMR, and mass spectroscopy were used to confirm the structures of compound III and α-aminophosphonate compounds IVa–f.
The FT-IR spectrum 3(2-amino-acetyl)-quinazolin-4(3H)-one, III, turned into characterized via way of means of the subsequent absorption bands (Fig.S1).
-
Band within 2968 cm-1 corresponds to CH2 aliphatic group.
-
The absorption band localized within 3043 cm-1 is due to CH aromatic stretching.
-
The stretching of C = CAromatic and C–CAromatic groups appears at 1613 and 1468 cm-1, respectively.
-
Bands inside 1705 cm-1 is because of exocyclic C = Oaliphatic, while a band at 1667 cm-1 is because of a C = O quinazoline ring.
-
A band at 1563 cm-1 is due to the C = N group.
-
Finally, the NH2 group is observed at 3155–3211 cm-1.
IR spectra of compounds (IVa-f) have been characterized using the subsequent absorption bands (Fig. 3).
-
Band within 1341–1388 cm-1 corresponds to stretching vibration of P = O moiety.
-
Band placed at 974–1069 cm-1 attributed to υ(P–O–C) and υ(P–CH) is absorbed at 762–772 cm-1.
-
The CH aromatic stretching band is absorbed at 3013–3268 cm-1, and CH aliphatic bands are seemed at 2852–3070 cm-1.
-
The stretching vibration C-CAr moiety is absorbed at 1438–1560 cm-1.
-
The bands placed inside 1655–1712 cm-1 are attributed to C = O stretching, while the C = N group is absorbed in 1560–1595 cm-1.
-
Finally, NH/OH corporations are absorbed at 3370-3439 cm-1 (Table S1, Fig S1).
1H-NMR (DMSO) spectrum of III was confirmed by the following signals:
-
The CH2 aliphatic protons produce a singlet signal at 2.52 ppm.
-
The NH2 group is responsible for the broad singlet at 9.16 ppm, while the aromatic protons are resonated as multiplet in the 7.22–8.77 ppm region (Fig.S2).
1H-NMR spectra of α-aminophosphonate derivatives, Iva–f, confirmed the subsequent signals:
-
CH2 aliphatic protons are responsible for a singlet signal at 2.50–2.2.53 ppm.
-
The CH–P proton causes a doublet signal around 4.95–5.95 ppm.
-
Multiplet signals at 5.96–8.3 ppm are attributed to multiplet aromatic protons, and the NH group is responsible for the singlet signal around 7.82–8.84 ppm (Table S2, Fig. S3).
The studied substances were characterized by elemental microanalysis, which revealed that the calculated value was extremely close to the estimated value. A molecular ion peak at m/z = 230 was confirmed in the mass spectrum of compound III (C10H9N3O2), and a molecular ion peak at m/z = 570 was confirmed in the mass spectrum of compound IVa (C29H23N4O7P). The fragmentation sample is proven in Scheme S1. Also, a molecular ion peak at m/z = 541 was validated in the mass spectrum of compound IVb (C29H24N3O6P), and a molecular ion peak at m/z = 557 is corresponding compound IVc (C29H24N3O7P) (Scheme S2, Fig.S4).
Biological studies
Antitumor activity
The anticancer impact of 3(2-amino-acetyl)-quinazolin-4(3H)-one, III, and diphenyl [(aryl) ((2-oxo-2-(4-oxo-quinazolin-3(4H)-yl) ethyl)amino) methyl] phosphonate, IVa-f, in vitro determined using the standard MTT method [51, 52] toward six consultant cell lines HePG-2, MCF-7, PC-3, HCT-116, Helaand Human lung fibroblast cell line, has been explained. Doxorubicin, a potent anticancer agent, is now a positive control. According to the obtained outcomes (Fig. 4), each synthesized α-aminophosphonate has good, mild, or vulnerable anti-proliferative efficacy against the six cell lines tested as follows:
-
1.
HePG-2: The compound IVc and IVf had a very high cytotoxicity activity, while the other compounds had moderate to mild cytotoxicity activity.
-
2.
HCT-116: compounds IVc, IVb and IVf showed a powerful inhibitory potency, while the rest of the compounds showed moderate to weak activity.
-
3.
PC-3: compound IVc and IVf demonstrated extraordinary anticancer activity, while the rest of the compounds showed moderate to weak activity.
-
4.
MCF-7: compound, IVc, showed an extreme inhibitory efficiency, while compounds IVb and IVb showed moderate activity. The rest of the compounds showed moderate to weak cytotoxicity activity.
-
5.
Hela: compound, IVc, showed extreme cytotoxicity activity, while the rest of the compounds showed moderate to weak activity.
-
6.
WI-38: compounds with a hydroxyl group, IVb, IVc and IVf, have low cytotoxicity activity on the normal cell, and the other compounds have moderate cytotoxicity activity on the normal cell.
From the above data, we could conclude that compound IVc which contains 2, 4-diOH and compound IVf, which contain 3-OMe and 4-OH substituent, have the most potent derivatives against the five cancer cell lines (Table 1).
Structure–activity relationship (SAR) of compounds, IVa-f
The experimental cytotoxicity of the examined compounds to their structures was used to postulate a structure–activity relationship of the produced α-aminophosphonate derivatives:
-
1.
The type of substituents in the aryl aldehyde moiety of α-amino phosphonates is essential for the wide range of cytotoxic activity against different cell lines (HePG-2, MCF-7, PC-3, HCT-116, Hela and WI-38).
-
2.
Compound IVc is the most potent toward the five cell lines and showed a medium cytotoxic activity against the normal lung cell WI-38, which can be attributed to two electron-donating (OH) groups compared with doxorubicin which has OH moieties.
-
3.
Because the number of OH moiety within the α-aminophosphonates ring system is reduced, compounds IVb and IVf are substantially less effective than compound IVc.
-
4.
Presence of electron-donating OH moiety in the α-aminophosphonate compounds IVb and IVf improves potency more than the other aminophosphonate compounds (Table 1).
Antioxidant activity using DPPH inhibition and erythrocyte hemolysis of α-amino phosphonates, IVa-f.
Using the DPPH radical scavenging method, the total radical scavenging capability of the studied compounds was measured and compared to ascorbic acid [53, 54] (Scheme 3).
The data showed that the compound, IVc, has the most potent antioxidant activity and sturdy inhibitory activity within the hemolysis assay of all compounds (Tables S3 and S4). All synthesized compounds have potent inhibitory activity within the hemolysis assay, except compound IVb, which exhibits a weak inhibitory activity within the hemolysis assay (Table S4). Thus, the presence of substituents within the phenyl-substituted α-amino phosphonates appears to have influenced the antioxidant screening of the investigated compounds. When combined with a DPPH solution, the novel di-substituted heterocyclic IVc-amino phosphonates can donate a hydrogen atom. As a result, the release of protons from NH groups of DPPH radicals is facilitated [55]. As a result, this molecule can protect cells and tissues against oxidative stress caused by unstable radicals, leading to various disorders, including cancer.
Quantum chemical calculations
Quantum chemistry approaches and molecular modeling procedures can define various molecular components, including reactivity, shape, binding residences, molecular fragments, and substituents. The relationship between structural factors and the organic activity of α-amino phosphonates was investigated using quantum chemical calculations. The optimized molecular systems with the lowest energies obtained from the computations of the researched compounds are demonstrated in Fig. 5.
Experimentally, it was discovered that compound IVc, which contains a di-substituent electron-donating OH groups within the aryl aldehyde moiety of the -amino phosphonates, had higher biological activity than the first quinazolinone compound, III, and the other phosphonates. Hence, quantum chemical calculations could be used for this rationalization. The compound IVc, which contains two OH groups, has the maximum HOMO energy (– 5.996 eV), indicating that it should react as a nucleophile (electron donor) and increase its donation capability to the target protein, resulting in increased activity (Table 2).
∆E is a characteristic reactivity descriptor; decreasing the value of ∆E increases the reactivity of the compounds. The calculations revealed that compound IVc has a lower separation energy (3.484 eV) than compound III (4.602 eV), as well as the alternative phosphonates, and so has the best softness values, which could explain the compound's reactivity (Table 2). On the other hand, ∆E is a crucial quantity for estimating the molecular system's nonlinear optical homes (NLO). A low ∆E cost indicated an easy digital transition and better NLO homes with an appreciation of a famous molecule, particularly urea [56]. The examined ∆E values for all molecules are lower than urea’s (6.884 eV), indicating that each of the produced phosphonates is a viable structure for photovoltaic devices such as solar cells. The most range of electronic loads (ΔNMAX) acquired from the environment (donor) through the inhibitor (acceptor) is the measured reactivity index through energy stabilization. The calculation showed that compound IVc has a higher ΔNmax, 2.442e, than that of compound III, 2.060e (Table 2), which shows that it permits the charge transfer and alternate of electron density among the compound and protein and hence growth of the biological activity that is in an excellent settlement with the experimental data. The calculations confirmed that di-substituted phosphonate, IVc, has a higher dipole moment value, 4.821D, than compound III, 2.067D, and mono-substituted phosphonate, IVb, 3.465D (Table 2). This method shows that this compound is more extraordinarily polarizable (hydrophilic) and hence more tremendously reactive than III and IVb; this is pretty much in line with the results of the experiments. It was concluded from the above discussion that the computation calculations showed that the α-aminophosphonate compounds increase the reactivity more than the starting quinazolinone compound, which agrees well with the experimental data. Also, the computed values can predict that di-substituted electron-donating OH groups within the aryl aldehyde moiety of α–amino phosphonates are significantly more effective than one OH group. These might be extra favorable for the reactivity in the direction of the enzyme and consents properly with the experimental data (Table 2).
The frontier molecular orbitals
Frontier molecular orbitals are incredibly beneficial as quantum chemical reactivity descriptors. The energy of the highest occupied molecular orbital (EHOMO) is associated with an electron-donating capability. In comparison, the energy of the lowest unoccupied molecular orbital (ELUMO) is strongly related to the prosperity of the molecule toward charge acceptance [57, 58]. HOMO and LUMO charge density distributions for α-aminophosphonate molecules are shown in Fig. 6. It changed into proven that, for electron-withdrawing substituted phosphonates, IVb, HOMO is in particular localized on the 2 phenyl groups connected to the phosphorous atom. The LUMO is mainly delocalized on the nitrophenyl moiety (Fig. 6). However, the HOMO for compound IVc is located on one of the phenyl groups attached to the phosphorous atom and at the dihydroxy-substituted phenyl moiety. Thus, it can interact with the biological target as a nucleophile (hydrogen bond donor). The LUMO of IVc is selectively delocalized at the quinazolinone ring, which can be interacted with the biological target as an electrophile (hydrogen bond acceptor). In the case of mono-electron-donating substituted phosphonates, IVb, the HOMO's charge density distribution is concentrated on one of the phenyl groups attached to the phosphorus atom, as well as on the hydroxy-substituted phenyl moiety (act as a nucleophile with the target enzyme) and LUMO is specifically delocalized at the quinazolinone ring (act as electrophile with the target enzyme) (Fig. 6).
Molecular electrostatic potential
The molecular electrostatic potential (MEP) is strongly influenced by electronic density. MEP is one of the most essential and valuable computational tools for revealing the most reactive regions for electrophilic and nucleophilic attacks [59, 60]. Different colors signify different values of the electrostatic potential on the surface. Red, orange, yellow, green, and blue indicate potential increases[61]. Red color regions of MEP represent the electrophilic reactive sites, while the nucleophilic reactive ones are represented by blue color (Fig. 6).
The negative ability covers the carbonyl moiety of the quinazolinone ring, the carbonyl moiety connected to the quinazoline ring, and the oxygen atoms connected to a phosphorous group. In comparison, the positive areas are above the remaining groups. The electrostatic interplay forces among the researched chemicals and the organic target can be defined from the MEP plots (Fig. 6).
Natural bond orbital analysis
The natural bond orbital (NBO) evaluation has become a powerful tool for studying intra- and intermolecular interactions and charge transfer and conjugative interactions within the molecular system[62]. The NBO evaluation becomes accomplished at the maximum active compound, IVc, with the use of the NBO 3.1 program [63] on the B3LYP/6-31G level in order to recognize various second-order interactions between the filled orbitals of one subsystem and the unoccupied orbitals of the other subsystems, which could indicate a degree of intramolecular hyperconjugation delocalization. The degree of interaction (energy stabilization) E (2) associated with electron delocalization I → j is envisioned as for each donor I and acceptor (j):
qi, Ei, Ej are orbital occupying, diagonal elements, and Fij, the off-diagonal Fock matrix. The extra in-depth interaction among electron donors and electron acceptors, the extra electrons-donating ability, and the greater the extent of conjugation of the entire system turned into related to the more significant stabilization energy E (2) value. The possible in-depth interactions for the maximum active compound IVc are tabulated in Table 3. The intramolecular charge transfer of the π-electron increases the molecule's polarity, making it charged for NLO characteristics. Also, the stabilization interactions among the LP orbitals and antibonding σ or π-orbitals are liable for the organic activity of a molecule and the feature function of pharmaceutical compounds [64]. Examining the Fock matrix in NBO basis using the second-order perturbation principle revealed substantial intramolecular hyper-conjugative interactions of -electrons, which drives the system's stability. The opposite conjugative interactions of π (C1–C2) → π*(C3–C4)/π*(C5–C6), (C3–C4) → π*(C1–C2)/π*(C5–C6) and π(C5–C6) → π*(C1–C2)/π*(C3–C4) are answerable for conjugation of the quinazoline ring's respective bonds (Table 3). Also, the maximum vital interaction (n–σ*) and (n–π*) energy, linked with the quinazoline moiety's internal resonance, is a donation of electrons LP (1) N7 → σ*(C5–C6)/σ*(C8–N9) with E (2) values 7. ninety–two and 17.37 kJ/mol, respectively, from LP (1) N9 → π*(N7–C8)/π*(C10–O11)/ π*(C12–O13) with, E(2) values 35.67, 44.41 and 37.96 kJ/mol, respectively, and from LP (2) O11 → σ*(C6–C10)/ σ*(N9–C10) with, E(2) values,17.41 and 32.21 kJ/mol, respectively. These increases the reactivity of quinazoline moiety. The most tremendous critical intramolecular hyper–conjugative interactions created by π-electrons (C20–C25) → π*(C21–C22)/π*(C23–C24),(C21–C22) → π*(C20–C25)/π*(C23–C24)andπ(C23–C24) → π*(C20-C25)/π*(C21–C22) confirmed stabilization energies (~ 25 kJ/mol), This resulted in an increase in electron delocalization withinside the substituted phenyl ring of α-aminophosphonate molecules. The orbital overlap between the n(O) and *σ(C–C) bond orbitals created the intramolecular hyper-conjugative interactions, which finally ends up withinside the intramolecular charge transfer ICT, resulting in the system’s stabilization. The sturdy intramolecular hyper-conjugative interaction from LP (2) O13 → σ*(N9–C12)/ σ*(C12–C14), which will increase the electron density ED (0.1075 and 0.0709e), respectively, and weakens the respective bonds, results in excessive stabilization, 32.31 and 18.18 kJ mol-1, respectively. Another research that looked at intramolecular hyper-conjugative interactions LP (3)O18 → σ*(C16–P17)/σ*(P17–O19)/σ*(P17–O33), that will increase the ED (0.179, 0.247, 0.269e), respectively, and weaken the respective bonds, results in excessive stabilization, 1.86, 17.73 and 41.51 kJ/mol, respectively. The stabilization primarily arises from the LP(1) O26 and LP(2) O26 to σ*(C22–C23) and π*(C23–C24) transitions, which might be chargeable for the α-aminophosphonate molecules' intermolecular charge transfer (ICT), 5.92 and 29.47 kJ/mol, respectively, which were validated as stabilization energies. Also, the interaction of LP (1)O40 prolonged to σ*(N15–H48) denotes a bigger delocalization and stabilization of the entire system. The different critical intramolecular conjugative interaction is tabulated in Table 3. From the NBO evaluation results, it can be inferred that within the α-amino phosphonates, the presence of di-substituted electron-donating OH groups will boost the molecule's reactivity.
Vibrational spectra
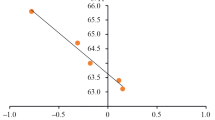
The examined compounds' vibrational frequencies were computed using DFT/B3LYP/6-31G. The investigated compounds' experimental and predicted FT-IR spectra are shown in Fig. 7 and Table 4. The experimentally observed wide band (3439–3370) cm-1 is due to symmetric stretching vibrations of the N–H group, whereas the calculated one is (3499–3246) cm-1. The band at (3286–3059) cm-1 is attributed to the characteristic aromatic C–H stretching, while the calculated one is at (3198–3158) cm-1. The calculated CH2 aliphatic stretching band is (2858–2802) cm-1, whereas the observed one is (2974–2852) cm-1. Also, the P = O group stretching vibration could be essential to research the α-amino phosphonates that are absorbed in (1388–1341) cm-1 (experimental) and at (1422–1411) cm-1 (calculated). The wide variety υ (P–O–C) becomes seemed at (1084–993) cm-1 (experimental) and (948–924) cm-1(calculated). Finally, the band appeared at (772–722) cm-1 (experimental) and (775–763) cm-1(estimated) because of υ (P–CH). The high correlation coefficient (R2 = 0.998) proves the remarkable concurrence between experimental and computed frequencies (Fig. 8). Moreover, the discrepancy in wave number values between experimental and predicted values can be explained by comparing theoretical calculations for the gaseous phase with actual results for the solid phase.
NMR spectral analysis
Nuclear Magnetic Resonance (NMR) spectroscopy has grown to be a vital research tool supplying accurate predictions of molecular geometries in present-day chemistry [65,66,67]. Computational NMR applications in diverse chemistry fields have been developed [68]. The computation of a few key NMR parameters, such as nuclear spin–spin couplings, shielding constants, and chemical shifts, is an active and essential part of theoretical research [69, 70]. The gauge-independent atomic orbital (GIAO) is a massive upgrade for the calculation of the NMR parameters Hartree–Fock (HF) method [71]. In this study, 1H-NMR chemical shift values of the quinazolinone, III and amino phosphonates, IVa-f, were calculated using HF/GIAO/6-31G method in DMSO solvent. Different NMR descriptors are listed in Table 5 [72]. Experimentally and theoretically generated 1H-NMR isotropic shift values had been as compared, and a linear correlation with a high correlation coefficient was observed (Fig. 9). A good correlation between experimental 1H-NMR chemical shift values and the GIAO-NMR approach was found.
Molecular docking results analysis for the most potent ligand, IVc
Molecular docking in the pharmaceutical enterprise is effective in silicon strategy for discovering novel therapies for unmet scientific desires predicting drug–target interactions. It no longer solely gives binding affinity between drugs and aims at the atomic level; however, it additionally elucidates the imperative pharmacological houses for specific medicines.
A molecular docking learn about used to be carried out to recognize the underlying mechanism of the examined molecule IVc toward MCF-7 breast most cancers mobile line. Molegro Virtual Docker (MVD) program performs binding ligand docking, so the most advantageous geometry of the ligand will be decided for the duration of the docking process. The 3D shape of the protein used to be acquired from the protein data bank using its unique (PDB code: 6p05) with a resolution of 1.54 Å [73]. A grid (x = 12.02, y = 13.71, z = 5.92 and r = 10 Å) is generated round the binding website already occupied through the co-crystallized ligand so that co-crystallized ligand can be excluded, and new compounds can be connected to the identities binding site of the target protein.
The co-crystallized YF2: N-{1-[1,1-di(pyridin-2-yl)ethyl]-6-(1-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-3-yl)-1H-indol-4-yl}ethanesulfonamide used to be chosen for trying out the validation of the docking method, Root-mean-square deviation (RMSD) price of docked poses of the native co-crystallized ligand is 1.4 Å and were clustered collectively, superimposition, as proven in Fig. 10. The low RMSD established that the molecular docking technique is validated for predicting the mode of motion of the examined molecule and the target enzyme. The drug YF2 fashioned 4 hydrogen bonds with Lys91, Asp88 and Asn140 of the binding pocket of the target receptor (Fig. 11).
The optimized molecular shape of the IVc molecule received from DFT/6-311G (d,p) was used as enter file for the docking calculations. The lowest energy docked conformer of IVc binds to the active website online residues with average binding energy, − 141.657 kcal/mol making intimate contacts with the active residence of the target enzyme thru significant bonded and nonbonded interactions. Suppose we disclose the binding mode of compound Ivc. It exhibited an appropriate docked score, − 23.021 kcal/mol, and formation of six hydrogen bonds with catalytic amino acids, i.e. Asp88, Tyr97, Lys91, Gln85 and Trp81with oxygen (O18, O26, O46) and nitrogen (N7 and N15) atoms (Fig. 12). Figure 13 confirms 2D interplay plot of ER + -IVc complicated visualized the usage of LIGPLOT + [73].
The hydrophobic phenyl moiety formed two pi-interactions with hydrophobic residues, Leu92 and Leu94, with distances 2.63 and 3.74 Å (Fig. 14). These interactions better the stabilization of ER + –inhibitor complicated and provide an explanation for the highest inhibition of IVc, which consents nicely with the experimental biological activity. Also, a strong electrostatic interplay is shaped between the electronegative oxygen atom (O26) and necessary positively charged residue, Lys91, with a distance of 2.25 Å. This might also be one of the structural prosperity for a higher activity of IVc (Fig. 15).
Figures 16 and 17 exhibit the surface illustration of the minimal energy shape and the secondary shape view of the docked pose of IVc in the binding web page of ER + .
Conclusion and outlook
Novel α-amino phosphonates-based quinazolinone rings were synthesized, and their structures were elucidated using different spectroscopic methods. The in vitro anticancer activities of diphenyl (aryl) [2-oxo-2-(4H-quinazolin-4-one-3-yl) ethyl-amino] methyl phosphonate, IVa-c, against six representative cell lines were explained. As a result, the di-substituted phosphonate compound, IVc, is the most active compound among all studied compounds. NBO analysis for IVc, the compound was calculated and confirmed that the presence of di-substituted electron-donating OH moieties will increase the reactivity of the compound.
The theoretical FT-IR data were calculated to discover the compounds' characteristic vibration frequencies, which correlate well with experimental data. Also, a good correlation was found between experimental 1H-NMR chemical shift values and the GIAO-NMR approach. Molecular docking calculation was performed for the most active molecule IVc using MVD software. The tested compound, IVc, could be a potent anticancer agent because it has a good docking score. Nitrogen, oxygen atoms and phenyl moieties form a hydrogen bond and hydrophobic and electrostatic interactions with crucial residues within the binding pocket. This study should assist in enhancing or finding new drugs for future anticancer agents.
References
A.R. Katritzky, Handbook of heterocyclic chemistry (Pergamon Press, New York, 1985)
J.A. Joule, K. Mills, Heterocyclic chemistry, 4th edn. (Blackwell, Oxford, UK, 2000)
R.S. Dua, S.K. Shrivastava, S.K. Sonwane, S. Shrivastava, Pharmacological significance of synthetic heterocycles scaffold: a review. Adv Biol Res. 5, 120–144 (2011)
M. Asif, Chemical characteristics, synthetic methods and biological potential of quinazoline and quinazolinone derivatives. Int. J. Med. Chem. 2014, 1–27 (2014). https://doi.org/10.1155/2014/395637
R. Gali, J. Banothu, M. Porika, R. Velpula, S. Hnamte, R. Bavantula, S. Abbagani, S. Busi, Indolylmethylene benzo[h] thiazolo [2,3-b] quinazolinones: Synthesis characterization and evaluation of anti-cancer and antimicrobial activities. Bioorg. Med. Chem. Lett. 24, 4239–4242 (2014). https://doi.org/10.1016/j.bmcl.2014.07.030
A. Moure, M. Orzaez, M. Sancho, A. Messeguer, Synthesis of enantiomerically pure perhydro -1,4- diazepine-2,5-dione and 1,4-piperazine-2,5-dione derivatives exhibiting potent activity as apoptosis inhibitors. Bioorg. Med. Chem. Lett. 22, 7097–7099 (2012). https://doi.org/10.1016/j.bmcl.2012.09.078
V. Chandregowda, A.K. Kush, R.G. Chandrasekara, Synthesis and invitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem. 44, 3046–3055 (2009). https://doi.org/10.1016/j.ejmech.2008.07.023
S.T. Al-Rashood, I.A. Aboldahab, M.N. Nagi, L.A. Abouzeid, A.A. Abdel-Aziz, S.G. Abdel-Hamide, K.M. Youssef, A.M. Al-Obaid, H.I. El-Subbagh, Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg. Med. Chem. 14, 8608–8621 (2006). https://doi.org/10.1016/j.bmc.2006.08.030
B. Insuasty, F. Orozco, C. Lizarazo, J. Quiroga, R. Abnia, M. Hursthouse, M. Nogueras, J. Cobo, Synthesis of new in deno[1,2-e] pyrimido[4,5-b] [1,4] diazepine-5,11-diones as potential antitumor agents. Bioorg. Med. Chem. 16, 8492–8500 (2008). https://doi.org/10.1016/j.bmc.2008.08.023
A.M. Alafeefy, S.I. Alqasoumi, A.E. Ashour, V. Masand, N.A. Al-Jaber, T.B. Hadda, M.A. Mohamed, Quinazo line-tyrphostin as a new class of antitumor agents, molecular properties prediction, synthesis and biological testing. Eur. J. Med. Chem. 53, 133–140 (2012). https://doi.org/10.1016/j.bmc.2008.08.023
L. Wu, C. Zhang, W. Li, Regioselective synthesis of 6-aryl benzo[h] [1,2,4]-triazolo[5,1-b] quinazoline-7,8-diones as potent antitumoral agents. Bioorg. Med. Chem. Lett. 23, 5002–5005 (2013). https://doi.org/10.1016/j.bmcl.2013.06.040
A. Kumar, P. Sharma, P. Kumari, B.L. Kalal, Exploration of antimicrobial and anti-oxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorg. Med. Chem. Lett. 21, 4353–4357 (2011). https://doi.org/10.1016/j.bmcl.2011.05.031
R. Rohini, R.P. Muralidhar, K. Shanker, A. Hu, V. Ravinder, Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c] quinazolines. Eur. J. Med. Chem. 45, 1200–1205 (2010). https://doi.org/10.1016/j.ejmech.2009.11.038
V. Jatav, S. Kashaw, P. Mishra, Synthesis and antimicrobial activity of some new 3–[5-(4-substituted) phenyl-1, 3, 4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med. Chem. Res. 17, 205–211 (2008)
Q. Ji, D. Yang, X. Wang, C. Chen, Q. Deng, Z. Ge, L. Yuan, X. Yang, F. Liao, Design, synthesis and evaluation of novel quinazoline-2,4-dione derivatives as chitin synthase inhibitors and antifungal agents. Bioorg. Med. Chem. 22, 3405–3413 (2014). https://doi.org/10.1016/j.bmc.2014.04.042
V.G. Ugale, S.B. Bari, Quinazolines: new horizons in anticonvulsant therapy. Eur. J. Med. Chem. 80, 447–501 (2014). https://doi.org/10.1016/j.ejmech.2014.04.072
H.I. El-Subbagh, G.S. Hassan, A.S. El-Azab, A.A.M. Abdel-Aziz, A.A. Kadi, A.M. Al-Obaid, O.A. Al-Shabanah, M.M. Sayed-Ahmed, Synthesis and anticonvulsant activity of some new thiazolo[3,2-a][1,3]diazepine, benzo[d] thiazolo [5,2-a] [12,6] diazepine and benzo [d] oxazolo[5,2-a][12,6] diazepine analogues. Eur. J. Med. Chem. 46, 5567–5572 (2011). https://doi.org/10.1016/j.ejmech.2011.09.021
L.D. Fader, S. Landry, S. Morin, S.H. Kawai, Y. Bousquet, O. Hucke, N. Goudreau, C.T. Lemke, P. Bonneau, S. Titolo, Optimization of a 1,5-dihydrobenzo[b][1,4] diazepine-2,4-dione series of HIV capsid assembly inhibitors 1: Addressing configurational instability through scaffold modification. Bioorg. Med. Chem. Lett. 23, 3396–3400 (2013). https://doi.org/10.1016/j.bmcl.2013.03.073
N. Zhang, P. Zhang, A. Baier, L. Cova, R.S. Hosmane, Dual inhibition of HCV and HIV by ring-expanded nucleosides containing the 5:7-fused imidazo [4,5-e] [1,3] diazepine ring system. In vitro results and implications. Bioorg. Med. Chem. Lett. 24, 1154–1157 (2014). https://doi.org/10.1016/j.bmcl.2013.12.121
H. Xiao, P. Li, D. Hu, B.A. Song, Synthesis and anti-TMV activity of novel ß-amino acid ester derivatives containing quinazoline and benzothiazole moieties. Bioorg. Med. Chem. Lett. 24, 3452–3454 (2014). https://doi.org/10.1016/j.bmcl.2014.05.073
A. Mucha, P. Kafarski, Ł Berlicki, Remarkable potential of the a-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 54, 5955–5980 (2011). https://doi.org/10.1021/jm200587f
W. Han, P. Mayer, A.R. Ofial, Iron-catalyzed oxidative mono-and bis-phosphonation of NN-dialkylanilines. Adv. Synth. Catal. 352, 1667–1676 (2010)
P. Kafarski, B. Lejczak, Amino phosphonic acids of potential medical importance. Curr. Med. Chem. 1, 301–312 (2001). https://doi.org/10.2174/1568011013354543
S.M. Agawane, J.M. Nagarkar, Nano ceria catalyzed synthesis of a-aminophosphonates under ultrasonication. Tetrahedron Lett. 52, 3499–3504 (2011). https://doi.org/10.1016/j.tetlet.2011.04.112
L. Pan, X.H. Liu, Y.X. Shi, B.L. Wang, S.H. Wang, Solvent and catalyst-free synthesis and antifungal activities of α-aminophosphonate containing cyclopropane moiety. Chem. Res. Chin. Univ. 26, 389–393 (2010)
S.A. Dake, D.S. Raut, K.R. Kharat, Ionic liquid promoted synthesis, antibacterial and in vitro anti-proliferative activity of novel a-aminophosphonate derivatives. Bioorg. Med. Chem. Lett. 21, 2527–2532 (2011). https://doi.org/10.1016/j.bmcl.2011.02.039
G.Y. Yao, M.Y. Ye, R.Z. Huang, Synthesis and antitumor activities of novel rhein-aminophosphonates conjugate. Bioorg. Med. Chem. Lett. 24, 501–507 (2014). https://doi.org/10.1016/j.bmcl.2013.12.030
G.S. Reddy, K.U.M. Rao, C.S. Sundar, Neat synthesis and anti-oxidant activity of a-aminophosphonates. Arab. J. Chem. 7, 833–838 (2014). https://doi.org/10.1016/j.arabjc.2013.01.004
N. Gangwar, V.K. Kasana, Tartaric acid-catalyzed synthesis of a-aminophosphonates undersolvent-free conditions. Synth. Commun. 41, 2800–2804 (2011). https://doi.org/10.1080/00397911.2010.515358
M.Y. Ye, G.Y. Yao, J.C. Wei, Synthesis, cytotoxicity DNA binding and apoptosis of rhein-phosphonate derivatives as antitumoragents. Int. J. Mol. Sci. 14, 9424–9439 (2013). https://doi.org/10.3390/ijms14059424
X.C. Huang, M. Wang, Y.M. Pan, Synthesis and antitumor activities of novel a-aminophosphonates dehydro abietic acid derivatives. Bioorg. Med. Chem. Lett. 23, 5283–5289 (2013). https://doi.org/10.1016/j.bmcl.2013.08.005
M. Ordóñez, A. Arizpe, F.J. Sayago, A.I. Jiménez, C. Cativiela, K. Fields, Practical and efficient synthesis of α-aminophosphonic acids containing 1,2,3,4-tetrahydroquinoline or 1,2,3,4-tetrahydroisoquinoline heterocycles. Molecules 21, 1–14 (2016). https://doi.org/10.3390/molecules21091140
A.X. Zhu, J. Zhang, S. Sun, Y. Guo, X. Zhu, J. Zhang, S. Sun, Y. Guo, S. Cao, Y. Zhao, Original article Synthesis and structure-activity relationships study of α -aminophosphonate derivatives containing a quinoline moiety. Chinese Chem. Lett. 28, 1514–1518 (2017). https://doi.org/10.1016/j.cclet.2017.02.012
T.E. Ali, S.M. El-edfawy, A convenient synthesis and biological evaluation of some novel linear and cyclic a -aminophosphonic acid derivatives containing a quinazolinone ring. Res Chem Intermed. 4, 1329–1347 (2016)
H. Luo, D. Hu, J. Wu, M. He, L. Jin, S. Yang, B. Song, Rapid synthesis and antiviral activity of ( Quinazolin-4-Ylamino ) methyl-phosphonates through microwave irradiation. Int. J. Mol. sci. 13, 6730–6746 (2012). https://doi.org/10.3390/ijms13066730
K.N. Houk, F. Liu, Holy grails for computational organic chemistry and biochemistry. Acc. Chem. Res. 50, 539–543 (2017). https://doi.org/10.1021/acs.accounts.6b00532
D.Y. Scherson, A.J. Shela, W. Jennifer, C.J. Brian, Surface structure and reactivity of rhodium oxide. J. Phys. Chem. 115, 11036–11044 (2011). https://doi.org/10.1021/jp110998e
J.J.P. Stewart, Optimization of parameters for semi-empirical methods I. Method. Comp. J. Chem. 10, 209 (1989). https://doi.org/10.1002/jcc.540100208
J.J.P. Stewart, Optimization of parameters for semi-empirical methods. II. Applications. Comp. J. Chem. 16, 221 (1989). https://doi.org/10.1002/jcc.540100209
M.M. Kabanda, L.C. Murulana, M. Ozcan, F. Karadag, Quantum chemical studies on the corrosion inhibition of mild steel by some triazoles and benzimidazole derivatives in acidic medium. Int. J. Electrochem. Sci. 7, 5035–5056 (2012)
P. Udhayakala, A. Jayanthi, T.V. Rajendiran, Adsorption and quantum chemical studies on the inhibition potentials of some formazan derivatives. Der Pharma Chem. 3, 528–539 (2011)
M.K. Awad, M.S. Masoud, M.A. Shaker, A.E. Ali, MP2 and DFT theoretical studies of the geometry, vibrational and electronic absorption spectra of 2-aminopyrimidine. Res Chem Intermed. 39, 2741–2761 (2013). https://doi.org/10.1007/s11164-012-0795-3
F.M. Atlam, M.K. Awad, E.A. El-bastawissy, Computational simulation of the effect of quantum chemical parameters on the molecular docking of HMG-CoA reductase drugs. J. Mol. Struct. 1075, 311–326 (2014). https://doi.org/10.1016/j.molstruc.2014.06.045
A. Ouahrouch, M. Taourirte, J.W. Engels, S. Benjelloun, H.B. Lazrek, Synthesis of New 1,2,3-triazol-4-yl-quinazoline nucleoside and acyclonucleoside analogues. Molecules 19, 3638–3653 (2014). https://doi.org/10.3390/molecules19033638
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G.Scalmani, V. Barone, B. Mennucci, G. A. Petersson and al. Gaussian 09, Revision C.01; Gaussian Inc.:Wallingford, CT, USA (2010).
A.D. Becke, Density-functional thermochemistry. II. The effect of the Perdew-Wang generalized-gradient correlation correction. J. Chem. Phys. 97, 9173–9177 (1992). https://doi.org/10.1063/1.464913
A.D. Becke, A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993). https://doi.org/10.1063/1.464304
C. Lee, W. Yang, R.G. Parr, Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. ReV. B. 37, 785–789 (1988). https://doi.org/10.1103/PhysRevB.37.785
R. Dennington, T. Keith, J. Millam, Gauss view, version 5 (Semichem Inc., Shawnee Mission, KS, 2009)
Molegro Virtual Docker(2008). http://www.molegro.com/mvd.product.php
T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63 (1983). https://doi.org/10.1016/0022-1759(83)90303-4
P.R. Twentyman, M. Luscombe, A study of some variables in a tetrazolium dye ( MTT ) based assay for cell growth and chemosensitivity. Br. J. Cancer. 56, 279–285 (1987)
M. Burits, F. Bucar, Anti-oxidant activity of Nigella sativa essential oil. Phytother. Res. 14, 323–328 (2000). https://doi.org/10.1002/1099-1573(200008)14:5%3c323::AID-PTR621%3e3.0.CO;2-Q
J. Ancerewicz, E. Migliavacca, P.A. Carrrupt, Structure–property relationships of trimetazidine derivatives and model compounds as potential anti-oxidants. Free Radical Biol. Med. 25, 113–120 (1998). https://doi.org/10.1016/S0891-5849(98)00072-0
N.M.M. Hamada, N.Y.M. Abdo, Synthesis, characterization, antimicrobial screening and free-radical scavenging activity of some novel substituted pyrazoles. Molecules 20, 10468–10486 (2015). https://doi.org/10.3390/molecules200610468
H. Yüksek, B. Göksu, S. Manap, M. Beytur, Ö. Gürsoy Kol, Synthesis of some new 4-[2-(2-methylbenzoxy)- benzylidenamino]-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives with their anti-oxidant properties. Int J Chem. 22, 1–29 (2018)
S. Anand, A. Muthusamy, Synthesis, characterization, electrochemical, electrical, thermal and ESIPT behaviour of oligobenzimidazoles of certain substituted benzimidazole carboxylic acids and their diode applications. J Mol Struct. 1177, 78–89 (2019). https://doi.org/10.1016/j.molstruc.2018.09.045
F.J. Luque, J.M. Lopez, M. Orozco, Perspective on electrostatic interactions of a solute with a continuum. a direct utilization of ab initio molecular potentials for the prevision of solvent effects. Theor Chem Acc. 103, 343–345 (2000). https://doi.org/10.1007/s002149900013
Y. Li, Y. Liu, H. Wang, X. Xiong, P. Wei, F. Li, Synthesis, crystal structure, vibration spectral, and DFT studies of 4-aminoantipyrine and its derivatives. Molecules 18, 877–893 (2013). https://doi.org/10.3390/molecules18010877
S. Moro, M. Bacilieri, C. Ferrari, G. Spalluto, Auto correlation of molecular electrostatic potential surface properties combined with partial least squares analysis as alternative attractive tool to generate ligand-based 3D-QSARs. Curr Drug Discov Technol. 2, 13–21 (2005). https://doi.org/10.2174/1570163053175439
F. Weinhold, C.R. Landis, Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pr. 2, 91–104 (2001). https://doi.org/10.1039/B1RP90011K
E.D. Glendening, Reed A.E., J.E. Carpenter, F. Weinhold, NBO Version 3.1.,TCI, Univ. Wisconsin, Madison., 65 (1998).
M. Amalanathan, J.I. Hubert, V.K. Rastogi, Molecular structure and vibrational spectral investigation of charge transfer NLO crystal naphthalene picrate for THz application. Spectrochim Acta A Mol Biomol Spectrosc. 108, 256–267 (2013). https://doi.org/10.1016/j.saa.2013.01.097
A.U. Rani, N. Sundaraganesan, M. Kurt, M. Cinar, M. Karabacak, FT-IR, FT-Raman, NMR spectra and DFT calculations on 4-chloro-N-methylaniline. Spectrochim Acta A Mol Biomol Spectrosc. 75, 1523–1529 (2010). https://doi.org/10.1016/j.saa.2010.02.010
N. Subramanian, N. Sundaraganesan, J. Jayabharathi, Molecular structure, spectroscopic (FT-IR, FT-Raman, NMR, UV) studies and first-order molecular hyperpolarizabilities of 1,2-bis(3-methoxy-4-hydroxybenzylidene)hydrazine by density functional method. Spectrochim Acta A Mol Biomol Spectrosc. 76, 259–269 (2010). https://doi.org/10.1016/j.saa.2010.03.033
L.G. Wade, Organic chemistry (Pearson Prentice Hall, New Jersey, 2006)
M. Buhl, T.V. Mourik, NMR spectroscopy: quantum-chemical calculations. WIREs Comput Mol Sci. 1, 634–647 (2011)
J. Vaara, J. Jokisaari, R.E. Wasylishen, Spin-spin coupling tensors as determined by experiment and computational chemistry. Prog Nucl Magn Reson Spectrosc. 41, 233–304 (2002). https://doi.org/10.1016/S0079-6565(02)00050-X
K. Jackowski, M. Jaszun’ski, Nuclear magnetic moments from spectra-experimental gas phase studies and nuclear shielding calculations. Conc Magn Reson A. 30, 246–260 (2007). https://doi.org/10.1002/cmr.a.20091
W.N. Lipscomb, The chemical shift and other second-order magnetic and electric properties of small molecules. Adv Magn Reson. 2, 137–176 (1966)
K. Wolinski, J.F. Hinton, P. Pulay, Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc. 112, 8251–8260 (1990). https://doi.org/10.1021/ja00179a005
Y. Li, J. Zhao, L.M. Gutgesell, Z. Shen, K. Ratia, K. Dye, O. Dubrovskyi, H. Zhao, F. Huang, D.A. Tonetti, G.R.J. Thatcher, R. Xiong, Novel pyrrolopyridone bromodomain and extra-terminal motif (BET) inhibitors effective in endocrine-resistant ER+ breast cancer with acquired resistance to fulvestrant and palbociclib. J Med Chem. 63, 7186–7210 (2020). https://doi.org/10.1021/acs.jmedchem.0c00456
A.C. Wallace, R.A. Laskowski, J.M.Thornto, LIGPLOT+. Protein Eng., (1995).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atlam, F.M., Hekal, H.A. Discovery of novel anticancer candidates based on a combination strategy of synthesis, characterization, biological activity evaluation, consensus docking and molecular modeling. J IRAN CHEM SOC 20, 1949–1973 (2023). https://doi.org/10.1007/s13738-023-02811-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02811-z