Abstract
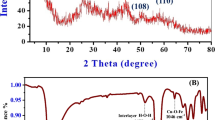
Here, the fabrication of a new electrode based on providing an electrocatalytic substrate by a newly assembled nanocomposition is reported. Ferric oxide-molybdenum disulfide-anthracite (Fe3O4–MoS2–ANT) modified glassy carbon electrode (GCE) was fabricated by a simple procedure and characterized by scanning electronic microscopy (SEM) and electrochemical impedance spectroscopy (EIS). The modified electrode was used as the sensing device for the voltammetric determination of paraquat (PQ), an important herbicide in environmental water resources. The sensor showed good electrocatalytic activity toward the reduction of PQ. We found that the electrochemical sensitivity and durability of the modified GCE enhance in the presence of ANT. Cyclic voltammetric (CV) and differential pulse voltammetry (DVP) were used and the results showed that the Fe3O4/MoS2/ANT composition provides excellent electrocatalytic response for the determination of PQ. The electrochemical responses of the ANT/MoS2/Fe3O4/GCE were investigated to find optimal conditions of scan rate, pH, type of supporting electrolyte, and response repeatability. A linear response of 0.5–180.0 µM with a correlation coefficient (R2) of 0.997 and a detection limit (LOD) of 0.03 µM of PQ were obtained under the optimized conditions. Reasonable recoveries of PQ were obtained between 93.33 and 101.95% for tested river water samples.











Similar content being viewed by others
References
R. Rajaram, L. Neelakantan, Recent advances in estimation of paraquat using various analytical techniques: A review. Result. Chem. 5, 100703–100719 (2023). https://doi.org/10.1016/j.rechem.2022.100703
Y. Huang, H. Zhan, P. Bhatt, S. Chen, Paraquat degradation from contaminated environments: current achievements and perspectives. Front. Microbiol. 10, 1754–1763 (2019). https://doi.org/10.3389/fmicb.2019.01754
Y.H. Jo, K.E. Kim, J.E. Rhee, G.J. Suh, W.Y. Kwon, S.H. Na, H.B. Alam, Therapeutic hypothermia attenuates acute lung injury in paraquat intoxication in rats. Resuscitation 82, 487–491 (2011). https://doi.org/10.1016/j.resuscitation.2010.11.028
C.M. Tanner, F. Kamel, G.W. Ross, J.A. Hoppin, S.M. Goldman et al., Rotenone, Paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872 (2011). https://doi.org/10.1289/ehp.1002839
F. Maya, J.M. Estela, V. Cerdà, Improved spectrophotometric determination of paraquat in drinking waters exploiting a multisyringe liquid core waveguide system. Talanta 85, 588–595 (2011). https://doi.org/10.1016/j.talanta.2011.04.022
H. Luo, X. Wang, Y. Huang, K. Lai, B.A. Rasco, Y. Fan, Rapid and sensitive surface-enhanced Raman spectroscopy (SERS) method combined with gold nanoparticles for determination of paraquat in apple juice. J. Sci. Food Agricult. 98, 3892–3898 (2018). https://doi.org/10.1002/jsfa.8906
M.L. Roldán, G. Corrado, O. Francioso, S. Sanchez-Cortes, Interaction of soil humic acids with herbicide paraquat analyzed by surface-enhanced Raman scattering and fluorescence spectroscopy on silver plasmonic nanoparticles. Anal. Chim. Acta 699, 87–95 (2011). https://doi.org/10.1016/j.aca.2011.05.001
R.D. Whitehead Jr., M.A. Montesano, N.K. Jayatilaka, B. Buckley, B. Winnik, L.L. Needham, D.B. Barr, Method for measurement of the quaternary amine compounds paraquat and diquat in human urine using high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 878, 2548–2553 (2010). https://doi.org/10.1016/j.jchromb.2009.09.029
Z. Zhao, F. Zhang, Z. Zhang, A facile fluorescent ‘turn-off’ method for sensing paraquat based on pyranine-paraquat interaction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 199, 96–101 (2018). https://doi.org/10.1016/j.saa.2018.03.042.
Y. Zhang, Y. Huang, L. Fu, J. Qiu, Z. Wang, A. Wu, Colorimetric detection of paraquat in aqueous and fruit juice samples based on functionalized gold nanoparticles. J. Food Compos. Anal. 92, 103574 (2020). https://doi.org/10.1016/j.jfca.2020.103574.
N. Lamei, M. Ezoddin, N.R. Kakavandi, K. Abdi, M. Ghazi-khansari, Ultrasound-assisted switchable solvent in determination of quaternary ammonium herbicide paraquat in biological, environmental water, and apple juice samples using chemical reduction process coupled to GC–MS detection. Chromatographia 81, 923–930 (2018). https://doi.org/10.1007/s10337-018-3500-x
E. Molaakbari, A. Mostafavi, H. Beitollahi, First electrochemical report for simultaneous determination of norepinephrine, Tyrosine and Nicotine using a nanostructure based sensor. Electroanalysis 26, 2252–2260 (2014). https://doi.org/10.1002/elan.201400338
Y. Zhang, G.M. Zeng, L. Tang, J. Chen, Y. Zhu, X.X. He, Y. He, Electrochemical sensor based on electrodeposited graphene-Au modified electrode and NanoAu carrier amplified signal strategy for attomolar mercury detection. Anal. Chem. 87, 989–996 (2015). https://doi.org/10.1021/ac503472p
E. Turker Acar, S. Ortaboy, G. Hisarl, G. Atun, Sensitive determination and electro-oxidative polymerization of azodyes on a carbon paste electrode modified with bentonite. Appl. Clay Sci. 105–106, 131–141 (2015). https://doi.org/10.1016/j.clay.2014.12.035.
A. Mohadesi, H. Beitollahi, Electrochemical and catalytic investigations of levodopa and folic acid by modified carbon nanotube paste electrode. Anal. Methods 3, 2562–2567 (2011). https://doi.org/10.1039/C1AY05344B
S. Ebrahimiasl, R. Seifi, R. Nahli, Z. Eftekhar, AzmiPpy/nanographene modified pencil graphite electrode nano sensor for detection and determination of herbicides in agricultural water. Sci. Adv. Mater. 9, 2045–2053 (2017). https://doi.org/10.1166/sam.2017.3110
X. Ye, Y. Gu, C. Wang, Fabrication of the Cu2O/polyvinyl pyrrolidone-graphene modified glassy carbon-rotating disk electrode and its application for sensitive detection of herbicide paraquat. Sens. Actuat. B 173, 530–539 (2012). https://doi.org/10.1016/j.snb.2012.07.047
A. Farahi, M. Achak, L. E-Gaini, M. A. El-Mhammedi, M. Bakasse, Electrochemical determination of paraquat in citric fruit based on electrodeposition of silver particles onto carbon paste electrode. J. Food Drug Anal. 23, 463–471 (2015). https://doi.org/10.1016/j.jfda.2015.03.003.
J. Zhang, Z. Lin, Y. Qin, Y. Li, X. Liu, Q. Li, H. Huang, Fabricated electrochemical sensory platform based on the boron nitride ternary nanocomposite film electrode for paraquat detection. ACS Omega 4, 18398–18404 (2019). https://doi.org/10.1021/acsomega.9b02658
F. Laghrib, M. Bakasse, S. Lahrich, M.A. El Mhammedi, Electrochemical sensors for improved detection of paraquat in food samples: a review. Mater. Sci. Eng. C 107, 11034 (2020). https://doi.org/10.1016/j.msec.2019.110349
F.Y. Kong, R.F. Li, L. Yao, Z.X. Wang, H.Y. Li, W.J. Wang, W. Wang, A novel electrochemical sensor based on Au nanoparticles/8-aminoquinoline functionalized graphene oxide nanocomposite for paraquat detection. Nanotechnology. 30, 285502 (2019). https://doi.org/10.1088/1361-6528/ab10ac.
J.A. Ribeiro, C.A. Carreira, H.J. Lee, F. Silva, A. Martins, C.M. Pereira, Voltammetric determination of paraquat at DNA-gold nanoparticle composite electrodes. Electrochim. Acta 55, 7892–7896 (2010). https://doi.org/10.1016/j.electacta.2010.03.058
P. Chuntib, S. Themsirimongkon, S. Saipany, J. Jakmunee, Sequential injection differential pulse voltammetric method based on screen printed carbon electrode modified with carbon nanotube/Nafion for sensitive determination of paraquat. Talanta 170, 1–8 (2017). https://doi.org/10.1016/j.talanta.2017.03.073
J. Liu, W. Fang, Z. Wei, Z. Qin, Z. Jiang, W. Shangguan, Metallic 1T-LixMoS2 co-catalyst enhanced photocatalytic hydrogen evolution over ZnIn2S4 floriated microspheres under visible light irradiation. Catal. Sci. Technol. 8, 1375–1382 (2018). https://doi.org/10.1039/C7CY02456H
A. Ambrosi, Z. Sofer, M. Pumera, Lithium intercalation compound dramatically influences the electrochemical properties of exfoliated MoS2. Small 11, 605–612 (2015). https://doi.org/10.1002/smll.201400401
Y. Zhou, C. Gao, Y.C. Guo, UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet-ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 6, 10286–10296 (2018). https://doi.org/10.1039/C8TA02679C
Y. Jiang, X. Li, S. Yu, L. Jia, X. Zhao, C. Wang, Reduced graphene oxide-modified carbon nanotube/polyimide film supported MoS2 nanoparticles for electrocatalytic hydrogen evolution. ADV. Funct. Mater. 25, 2693–2700 (2015). https://doi.org/10.1002/adfm.201500194
N. Lingappan, N.H. Van, S. Lee, D.J. Kang, Growth of three-dimensional flower-like molybdenum disulfide hierarchical structures on graphene/carbon nanotube network: an advanced heterostructure for energy storage devices. J. Power Sourc. 280, 39–46 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.064
X. Zhang, P. Ding, Y. Sun, Y. Wang, Y. Wu, J. Guo, Shell-core MoS2 nanosheets@Fe3O4 sphere heterostructure with exposed active edges for efficient electrocatalytic hydrogen production. J. Alloys Compounds 715, 53–59 (2017). https://doi.org/10.1016/j.jallcom.2017.04.315
Y. Zhang, P. Chen, F.F. Wen, B. Yuan, H. Wang, Fe3O4 nanospheres on MoS2 nanoflake: Electrocatalysis and detection of Cr(VI) and nitrite. J. Electroanal. Chem. 761, 14–20 (2016). https://doi.org/10.1016/j.jelechem.2015.12.004
C.L. Han, G. Huang, D. Zhu, K. Hu, Facile synthesis of MoS2/Fe3O4 nanocomposite with excellent Photo-Fenton-like catalytic performance. Mater. Chem. Phys. 200, 16–22 (2017). https://doi.org/10.1016/j.matchemphys.2017.07.065
Z. Li, Y. Zhang, W. Zhang, Controlled synthesis of CNTs/MoS2/Fe3O4 for high-performance supercapacitors. Mater. Res. Express 4, 055018 (2017). https://doi.org/10.1088/2053-1591/aa6c3f.
M.M. Maroto-Valer, Z. Tang, Y. Zhang, CO2 capture by activated and impregnated anthracites. Fuel Proc. Technol. 86, 1487–1502 (2005). https://doi.org/10.1016/j.fuproc.2005.01.003
D. Lozano-Castelló, M.A. Lillo-Ródenas, D. Cazorla-Amorós, A. Linares-Solano, Preparation of activated carbons from Spanish anthracite: I. Activation by KOH. Carbon 39, 741–749 (2001). https://doi.org/10.1016/S0008-6223(00)00185-8
H. Parham, B. Zargar, F. Khoshnam, Ultrasonic-assisted solid-phase extraction pre-concentration and determination of nicotinamide and nicotinic acid by high-performance liquid chromatography using anthracite extraction. Food. Anal. Methods 8, 2235–2242 (2015). https://doi.org/10.1007/s12161-015-0095-9
H. Parham, S. Saeed, Pre-concentration and determination of traces of nitrobenzene and 1,3-dinitrobenzene in water samples using anthracite adsorbent. J. Indust. Engin. Chemistry 20, 1003–1009 (2014). https://doi.org/10.1016/j.jiec.2013.06.035
M.H. Liao, D.H. Chen, Preparation and characterization of a novel magnetic nano-adsorbent. J. Mater. Chem. 12, 3654–3659 (2002). https://doi.org/10.1039/B207158D
L. Carlos, S. de F-Filho, V. Santos, Differential pulse voltammetric determination of paraquat using a bismuth-film electrode. Electroanalysis 22 , 1260–1266 (2010) . https://doi.org/10.1002/elan.200900553.
T. Paramalinggam, A.R.M. Yusoff, M.S. Qureshi, Z.A. Shah, P. Sathishkumar, Z. Yusop, M. Khalid, F.M. Khokhar, Determination of Paraquat dichloride from water samples using differential pulse cathodic stripping voltammetry. Russ. J. Electrochem. 54, 1155–1163 (2018). https://doi.org/10.1134/S1023193518140069
A. Savary, A. Yari, Determination of the herbicide paraquat using the new Ag-GO/CuO/GCE-modified glassy carbon electrode by differential pulse voltammetry. J. Chin. Chem. Soc. 1, 1–11 (2021). https://doi.org/10.1002/jccs.202100094
M. Ma, Y. Zhang, W. Yu, H. Shen, H. Zhang, N. Gu, Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A 212, 219–226 (2003). https://doi.org/10.1016/S092741
D. De Souza, S.A.S. Machado, Electrochemical detection of the herbicide paraquat in natural water and citric fruit juices using microelectrodes. Anal. Chem. Acta 546, 85–91 (2005). https://doi.org/10.1016/j.aca.2005.05.020
I.C. Lopes, D.D. Souza, S.A.S. Machado, A.A. Tanaka, Voltammetric detection of paraquat pesticide on a phthalocyanine-based pyrolitic graphite electrode. Anal. Bioanal. Chem. 388, 1907–1914 (2007). https://doi.org/10.1007/s00216-007-1397-6
T.G. Diaz, A.G. Cabanillas, F. Salinas, Square-wave and differential pulse oxidative voltammetric determination of diquat and paraquat in alkaline medium. Electroanalysis 12, 616 (2000). https://doi.org/10.1002/(SICI)15214109(200005)12:8%3c616::AID-ELAN616%3e3.0.CO;2-X
Z. Pourakbari, M. Sheykhan, Al.Aliakbar, A new poly carboxylic catex polymer-gold nanoparticles modified electrode for determination of paraquat by voltammetry method. J. Environ. Chem. Engin. 8, 104284 (2020). https://doi.org/10.1016/j.jece.2020.104284.
L.L.C. Garcia, L.C.S. Figueiredo-Filho, G.G. Oliveira, O. Fatibello-Filho, C.E. Banks, Square-wave voltammetric determination of paraquat using a glassy carbon electrode modified with multiwalled carbon nanotubes within a dihexadecyl hydrogen phosphate (DHP) film. Sens. Actuat. B 181, 306–311 (2013). https://doi.org/10.1016/j.snb.2013.01.091
Acknowledgements
We sincerely thank Dr. Mohsen Adeli and his team for their effective assistance in the synthesis of nanocomposites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yari, A., Savari, A. Fabrication of a highly sensitive new ANT/MoS2/Fe3O4/GCE nanocomposite electrochemical sensor for herbicide Paraquat residual in environmental water resources. J IRAN CHEM SOC 20, 1939–1948 (2023). https://doi.org/10.1007/s13738-023-02810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02810-0