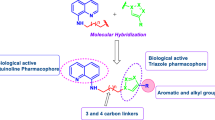
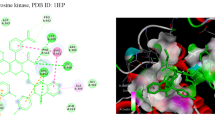
Abstract
In order to combat microbial infections and to address the issue of multi-drug resistance, a class of novel piperidine-bearing cinnamic acid hybrids 4a–4l were synthesized and validated using various spectroscopic techniques like IR, NMR, and mass spectrometry. In addition, the compounds were assessed for antimicrobial activity. Compound 4l demonstrated significant activity against Pseudomonas aeruginosa and Escherichia coli strains with MIC = 50 and 12.5 µg/mL, and compounds 4a, 4d, 4e, and 4l exhibited considerable activity against all fungal strains ranging from MFC = 125–250 µg/mL. An in silico ADMET study indicated that most compounds show favorable drug-like and toxicological properties. Furthermore, a molecular docking study revealed that compounds 4d, 4e, 4h, and 4j could be lodged in the active pocket and inhibit human fungal Candida albicans Hsp90 NBD protein via various interactions, and results indicated the synthesized analogs to be promising lead compounds in the search for novel antifungal drug-like molecules to be orally bioavailable.
Graphical abstract






Similar content being viewed by others
References
E.G. Brown, Ring nitrogen and key biomolecules. Ring Nitrogen Key Biomol. (1998). https://doi.org/10.1007/978-94-011-4906-8
G. Facchetti, I. Rimoldi, Anticancer platinum(II) complexes bearing N-heterocyclerings. Bioorg. Med. Chem. Lett. 29, 1257–1263 (2019). https://doi.org/10.1016/J.BMCL.2019.03.045
X. Liang, L. Zhang, F. Li, S. Luan, C. He, L. Yin, Z. Yin, Y. Zou, G. Yue, L. Li, X. Song, C. Lv, W. Zhang, B. Jing, Autophagy-regulating N-heterocycles derivatives as potential anticancer agents. Future Med. Chem. 12, 223–242 (2019). https://doi.org/10.4155/FMC-2019-0294
K.B. Patel, P. Kumari, Anticancer activity and docking study of flavone derivatives as peroxisome proliferator-activated receptorγ inhibitors. Struct. Chem. (2022). https://doi.org/10.1007/S11224-022-01926-Y
A.A. Shaddy, A.A. Kamel, W.M. Abdou, Synthesis, quantitative structure-activity relationship, and anti-inflammatory profiles of substituted 5- and 6-N-heterocycle bisphosphonate esters. Synth. Commun. 43, 236–252 (2012). https://doi.org/10.1080/00397911.2011.595603
N. Desideri, R. Fioravanti, L.P. Monaco, E.M. Atzori, A. Carta, I. Delogu, G. Collu, R. Loddo, Design, synthesis, antiviral evaluation, and sar studies of new 1-(phenylsulfonyl)-1H-pyrazol-4-yl-methylaniline derivatives. Front. Chem. 7, 214 (2019). https://doi.org/10.3389/FCHEM.2019.00214
R. Ardeleanu, A. Dascălu, S. Shova, A. Nicolescu, I. Roşca, B.I. Bratanovici, V. Lozan, G. Roman, 4′-(2H-tetrazol-5-yl)-[1,1′-biphenyl]-4-carboxylic acid: Synthetic approaches, single crystal X-ray structures and antimicrobial activity of intermediates. J. Mol. Struct. 1173, 63–71 (2018). https://doi.org/10.1016/J.MOLSTRUC.2018.06.086
Prachayasittikul, S., Worachartcheewan, A., & Lawung, R. Activities ofthiotetrahydropyridines asantioxidantandantimicrobialagents. (2009). https://doi.org/10.17877/DE290R-14217
Y. Zhou, V.E. Gregor, B.K. Ayida, G.C. Winters, Z. Sun, D. Murphy, G. Haley, D. Bailey, J.M. Froelich, S. Fish, S.E. Webber, T. Hermann, D. Wall, Synthesis and SAR of 3,5-diamino-piperidine derivatives: novel antibacterial translation inhibitors as aminoglycoside mimetics. Bioorg. Med. Chem. Lett. 17, 1206–1210 (2007). https://doi.org/10.1016/J.BMCL.2006.12.024
R. Aeluri, M. Alla, V.R. Bommena, R. Murthy, N. Jain, Synthesis and antiproliferative activity of polysubstituted tetrahydropyridine and piperidin-4-one-3-carboxylate derivatives. Asian J. Org. Chem. 1, 71–79 (2012). https://doi.org/10.1002/AJOC.201200010
A. Ravindernath, M.S. Reddy, Synthesis and evaluation of anti-inflammatory, antioxidant and antimicrobial activities of densely functionalized novel benzo [d] imidazolyl tetrahydropyridine carboxylates. Arab. J. Chem. 10, S1172–S1179 (2017). https://doi.org/10.1016/J.ARABJC.2013.02.011
A. Mochizuki, Y. Nakamoto, H. Naito, K. Uoto, T. Ohta, Design, synthesis, and biological activity of piperidine diamine derivatives as factor Xa inhibitor. Bioorg. Med. Chem. Lett. 18(2), 782–787 (2008). https://doi.org/10.1016/J.BMCL.2007.11.037
R.S. Dawood, S.A. Dayl, Synthesis, identification and molecular docking studies of N-functionalized piperidine derivatives linked to 1,2,3-triazole ring. Synth. Commun. 50, 2422–2431 (2020). https://doi.org/10.1080/00397911.2020.1776876
A.B. Patel, K.H. Chikhalia, P. Kumari, Access to antimycobacterial and anticancer potential of some fused quinazolines. Res. Chem. Intermed. 41, 4439–4455 (2015). https://doi.org/10.1007/S11164-014-1542-8
A.B. Patel, K.H. Chikhalia, P. Kumari, Study of new β-lactams-substituted s-triazine derivatives as potential bioactive agents. Med. Chem. Res. 24, 468–481 (2019). https://doi.org/10.1007/S00044-014-1151-5
D. Patel, P. Kumari, N.B. Patel, Synthesis and biological evaluation of coumarin based isoxazoles, pyrimidinthiones and pyrimidin-2-ones. Arab. J. Chem. 10, S3990–S4001 (2017). https://doi.org/10.1016/J.ARABJC.2014.06.010
R.V. Patel, P. Kumari, D.P. Rajani, K.H. Chikhalia, Synthesis, characterization and pharmacological activities of 2-[4-cyano-(3-trifluoromethyl)phenyl amino)]-4-(4-quinoline/coumarin-4-yloxy)-6-(fluoropiperazinyl)-s-triazines. J. Fluor. Chem. 132, 617–627 (2011). https://doi.org/10.1016/J.JFLUCHEM.2011.06.021
G.D. Brown, D.W. Denning, N.A.R. Gow, S.M. Levitz, M.G. Netea, T.C. White, Hidden killers: human fungal infections. Sci. Transl. Med. (2012). https://doi.org/10.1126/SCITRANSLMED.3004404
M.A. Pfaller, D.J. Diekema, Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163 (2007). https://doi.org/10.1128/CMR.00029-06
N. Robbins, G.D. Wright, L.E. Cowen, Antifungal drugs: the current armamentarium and development of new agents. Microbiol. Spectr. (2016). https://doi.org/10.1128/MICROBIOLSPEC.FUNK-0002-2016
M.C. Fisher, N.J. Hawkins, D. Sanglard, S.J. Gurr, Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742 (2018). https://doi.org/10.1126/SCIENCE.AAP7999
A. Makowska, F. Saczewski, P.J. Bednarski, J. Saczewski, Ł Balewski, Hybrid molecules composed of 2,4-diamino-1,3,5-triazines and 2-imino-coumarins and coumarins. Synthesis and cytotoxic properties. Molecules 23, 1616 (2018). https://doi.org/10.3390/MOLECULES23071616
H. Seyrani, S. Ramezanpour, A. Vaezghaemi, F. Kobarfard, A sequential Ugi–smiles/transition-metal-free endo-dig Conia–ene cyclization: the selective synthesis of saccharin substituted 2,5-dihydropyrroles. New J. Chem. 45, 15647–15654 (2021). https://doi.org/10.1039/D1NJ01159F
J.S. Lan, J.W. Hou, Y. Liu, Y. Ding, Y. Zhang, L. Li, T. Zhang, Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 32, 776–788 (2018). https://doi.org/10.1080/14756366.2016.1256883
X.T. Xu, X.Y. Deng, J. Chen, Q.M. Liang, K. Zhang, D.L. Li, P.P. Wu, X. Zheng, R.P. Zhou, Z.Y. Jiang, A.J. Ma, W.H. Chen, S.H. Wang, Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 189, 112013 (2020). https://doi.org/10.1016/J.EJMECH.2019.112013
G.D.A. de Lima, M.P. Rodrigues, T.A.O. de Mendes, G.A. Moreira, R.P. Siqueira, A.M. da Silva, B.G. Vaz, J.L.R. Fietto, G.C. Bressan, M. Machado-Neves, R.R. Teixeira, Synthesis and antimetastatic activity evaluation of cinnamic acid derivatives containing 1,2,3-triazolic portions. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 53, 1–9 (2018). https://doi.org/10.1016/J.TIV.2018.07.015
I. Perković, S. Raić-Malić, D. Fontinha, M. Prudêncio, L. Pessanha de Carvalho, J. Held, T. Tandarić, R. Vianello, B. Zorc, Z. Rajić, Harmicines−harmine and cinnamic acid hybrids as novel antiplasmodial hits. Eur. J. Med. Chem. 187, 111927 (2020). https://doi.org/10.1016/J.EJMECH.2019.111927
J.D. Guzman, Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 19, 19292–19349 (2014). https://doi.org/10.3390/MOLECULES191219292
A.R. Zala, D.P. Rajani, I. Ahmad, H. Patel, P. Kumari, Synthesis, characterization, molecular dynamic simulation, and biological assessment of cinnamates linked to imidazole/benzimidazole as a CYP51 inhibitor. J. Biomol. Struct. Dyn. (2023). https://doi.org/10.1080/07391102.2023.2170918
B.F. Ruan, W.W. Ge, H.J. Cheng, H.J. Xu, Q.S. Li, X.H. Liu, Resveratrol-based cinnamic ester hybrids: synthesis, characterization, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 32, 1282–1290 (2017). https://doi.org/10.1080/14756366.2017.1381090
B. Korošec, M. Sova, S. Turk, N. Kraševec, M. Novak, L. Lah, J. Stojan, B. Podobnik, S. Berne, N. Zupanec, M. Bunc, S. Gobec, R. Komel, Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 116, 955–966 (2014). https://doi.org/10.1111/JAM.12417
B. Podobnik, J. Stojan, L. Lah, N. Kraševec, M. Seliškar, T.L. Rižner, D. Rozman, R. Komel, CYP53A15 of Cochliobolus lunatus, a target for natural antifungal compounds. J. Med. Chem. 51, 3480–3486 (2008). https://doi.org/10.1021/JM800030E
A.R. Zala, D.P. Rajani, P. Kumari, Design, synthesis, molecular docking and antimicrobial and antimycobacterial activities of novel hybrid of coumarin-cinnamic acids. Chem. Data Collect. 39, 100862 (2022). https://doi.org/10.1016/J.CDC.2022.100862
A.R. Zala, D.P. Rajani, P. Kumari, Synthesis, molecular docking, ADME study, and antimicrobial potency of piperazine based cinnamic acid bearing coumarin moieties as a DNA gyrase inhibitor. J. Biochem. Mol. Toxicol. (2022). https://doi.org/10.1002/jbt.23231
K. Drlica, X. Zhao, DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61(3), 377–392 (1997). https://doi.org/10.1128/MMBR.61.3.377-392.1997
S.M. Hammond, 3 biological activity of polyene antibiotics. Prog. Med. Chem. 14(1), 105–179 (1977). https://doi.org/10.1016/S0079-6468(08)70148-6
Y. Kiat, Y. Vortman, N. Sapir, Feather moult and bird appearance are correlated with global warming over the last 200 years. Nat. Commun. 10, 1–7 (2019). https://doi.org/10.1038/s41467-019-10452-1
R.A. Friesner, J.L. Banks, R.B. Murphy, T.A. Halgren, J.J. Klicic, D.T. Mainz, M.P. Repasky, E.H. Knoll, M. Shelley, J.K. Perry, D.E. Shaw, P. Francis, P.S. Shenkin, Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004). https://doi.org/10.1021/JM0306430
T.A. Halgren, R.B. Murphy, R.A. Friesner, H.S. Beard, L.L. Frye, W.T. Pollard, J.L. Banks, Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47, 1750–1759 (2004). https://doi.org/10.1021/JM030644S
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne, The protein data bank. Nucleic Acids Res. 28, 235–242 (2000). https://doi.org/10.1093/NAR/28.1.235
C. Tang, Y. Ye, Y. Feng, R.J. Quinn, TCM, brain function and drug space. Nat. Prod. Rep. 33, 6–25 (2015). https://doi.org/10.1039/C5NP00049A
D.G. Levitt, M.D. Levitt, Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 9, 229–255 (2016). https://doi.org/10.2147/IJGM.S102819
Acknowledgements
The authors thank Microcare Laboratory-Surat, Gujarat, India, for performing in vitro antimicrobial activity and IISc, Bangalore, India for providing spectral data of synthesized compounds. The authors are also thankful to Schrödinger Inc. for providing the demo license of Schrödinger Suite that has tremendously helped in the computational study. The authors are thankful to the Department of Chemistry of S.V. National Institute Technology, Surat, Gujarat, India, for providing all the facilities for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest was reported by the author(s).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zala, A.R., Rajani, D.P. & Kumari, P. Design, synthesis, molecular docking and in silico ADMET investigations of novel piperidine-bearing cinnamic acid hybrids as potent antimicrobial agents. J IRAN CHEM SOC 20, 1843–1856 (2023). https://doi.org/10.1007/s13738-023-02801-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02801-1