Abstract
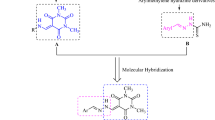
Aryl hydrazones bearing thiazolyl coumarin hybrids 1–32 were prepared by following 'one-pot' two-steps reaction scheme. Various arylaldehydes were reacted to thiosemicarbazide under acidic condition to form aryl thiosemicarbazone intermediates which in turn treated with 3-bromoacetyl coumarin under basic condition to afford thiazolyl coumarin hybrids 1–32. All hybrids were recognized by EI- and HREI-MS and 1H- and 13C-NMR spectroscopic techniques. Compounds 1–32 were screened for in vitro inhibitory activity against urease enzyme and displayed good to moderate inhibitory potential in the ranges of IC50 = 16.29 ± 1.1–256.30 ± 1.4 µM. Worth stating that compound 21 (IC50 = 16.29 ± 1.1 µM) was identified as more potent urease inhibitor than the standard acetohydroxamic acid (IC50 = 27.0 ± 0.5 µM). Derivatives 19 (IC50 = 77.67 ± 1.5 µM) and 30 (IC50 = 71.21 ± 1.6 µM) were found to be moderately active. Structure–activity relationship revealed that -F, -Cl, -OH, and -OMe groups and their respective positions on aryl ring are playing important role in urease enzyme inhibition. Molecular docking studies identified important interaction between the ligand (active hybrids) and urease active site.








Similar content being viewed by others
References
B. Krajewska, Ureases I. Functional, catalytic and kinetic properties: a review. J. Mol. Cat. B: Enz. 59, 9–21 (2009)
B. Krajewska, Ureases: roles, properties and catalysis. Wiadomości Chemiczne 56, 223–253 (2002)
J.B. Sumner, The isolation and crystallization of the enzyme urease preliminary paper. J. Biol. Chem. 69, 435–441 (1926)
M.M. Ahari-Mostafavi, A. Sharifi, M. Mirzaei, M. Amanlou, Novel and versatile methodology for synthesis of β-aryl-β-mercapto ketone derivatives as potential urease inhibitors. J. Iran. Chem. Soc. 11, 1113–1119 (2014)
L. Asadi, K. Gholivand, K. Zare, Phosphorhydrazides as urease and acetylcholinesterase inhibitors: biological evaluation and QSAR study. J. Iran. Chem. Soc. 13, 1213–1223 (2016)
M.A. Lodhi, U. Ashiq, R.A. Jamal, K.M. Khan, Study of vanadium complexes as novel inhibitors of Bacillus pasteurii and Canavalia ensiformis urease enzyme. J. Chem. Soc. Pak. 37, 549–558 (2015)
L. Holm, C. Sander, An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Prot. Struc. Func. Bioinf. 28, 72–82 (1997)
C. Belzer, J. Kusters, E. Kuipers, A.V. Vliet, Urease induced calcium precipitation by Helicobacter species may initiate gallstone formation. Gut 55, 1678–1679 (2006)
D.P. Griffith, D. Musher, C. Itin, Urease. The primary cause of infection-induced urinary stones. Invest. Urol. 13, 346–359 (1976)
S. Wahid, S. Jahangir, M.A. Versiani, K.M. Khan, U. Salar, M. Ashraf, U. Farzand, A. Wadood, M. Taha, S. Perveen, Atenolol thiourea hybrid as potent urease inhibitors: Design, biology oriented drug synthesis, inhibitory activity screening, and molecular docking studies. Bioorg. Chem. 94, 103359 (2020)
F. Seraj, Kanwal, K.M. Khan, A. Khan, M. Ali, R. Khalil, Z. Ul‑Haq, S. Hameed, M. Taha, U. Salar, S. Perveen. Biology‑oriented drug synthesis (BIODS), in vitro urease inhibitory activity, and in silico studies on ibuprofen derivatives. Mol. Diver. (2020) 1–15.
S. Vosooghi, M. Farzipour, N.B. Saeedi, M. Shareh, S. Mahdavi, A. Mahernia, M. Foroumadi, A. Amanlou, Shafiee, Synthesis of novel 5-arylidene (thio) barbituric acid and evaluation of their urease inhibitory activity. J. Iran. Chem. Soc. 12, 1487–1491 (2015)
J.M. Bremenr, Recent research on problems in the use of urea as a nitrogen fertilizer. Fertilizer Res. 42, 321–329 (1995)
M.A.J. Dominguez, C. Sanmartin, M. Font, J.A. Palop, S.S. Francisco, O. Urrutia, F. Houdusse, J.M.G. Mina, Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors. J. Agri. Food Chem. 56, 3721–3731 (2008)
G. Mohiuddin, K.M. Khan, U. Salar, M.A. Kanwal, A. Lodhi, M. Wadood, S. Perveen. Riaz, Biology-oriented drug synthesis (BIODS), in vitro urease inhibitory activity, and in silico study of S-naproxen derivatives. Bioorg. Chem. 83, 29 (2019)
J.R.S. Hoult, M. Payá, Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen. Pharmacol. 27, 713–722 (1996)
M.A. Musa, J.S. Cooperwood, M.O.F. Khan, A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 15, 2664–2679 (2008)
X.-M. Peng, G.L.V. Damu, H. Zhou, Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 19, 3884–3930 (2013)
K.V. Sashidhara, A. Kumar, M. Kumar, J. Sarkar, S. Sinha, Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 20, 7205–7211 (2010)
I. Kempen, D. Papapostolou, N. Thierry, L. Pochet, S. Counerotte, B. Masereel, J.M. Foidart, M. Reboud-Ravaux, A. Noël, B. Pirotte, 3-Bromophenyl 6-acetoxymethyl-2-oxo-2H-1-benzopyran-3-carboxylate inhibits cancer cell invasion in vitro and tumour growth in vivo. Br. J. Cancer 88, 1111–1118 (2003)
C.A. Kontogiorgis, D.J. Hadjipavlou-Litina, Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg. Med. Chem. Lett. 14, 611–614 (2004)
F. Rahim, H. Samreen, M.I. Ullah, U. Fakhri, S. Salar, K.M. Perveen, M.I. Khan, Choudhary, Anti-leishmanial activities of synthetic biscoumarins. J. Chem. Soc. Pak. 39, 79–82 (2017)
U. Salar, K.M. Khan, A. Jabeen, A. Faheem, M. Taha, F. Ali, S. Syed, S.M. Haider, S. Perveen, Anti-inflammatory activity of 3-thiazolyl coumarins. J. Chem. Soc. Pak. 39, 578–585 (2017)
T. Ma, L. Liu, H. Xue, L. Li, C. Han, L. Wang, Z. Chen, G. Liu, Chemical library and structure-activity relationships of 11-demethyl-12-oxo calanolide A analogues as anti-HIV-1 agents. J. Med. Chem. 51, 1432–1446 (2008)
A.G. Kidane, H. Salacinski, A. Tiwari, K.R. Bruckdorfer, A.M. Seifalian, Anticoagulant and antiplatelet agents: their clinical and device application(s) together with usages to engineer surfaces. Biomacromol 5, 798–813 (2004)
U. Salar, K.M. Khan, A. Jabeen, A. Faheem, M.I. Fakhri, S.M. Saad, S. Perveen, M. Taha, A. Hammed, Coumarin sulfonates: as potential leads for ROS inhibition. Bioorg. Chem. 69, 34–47 (2016)
K.M. Khan, Z.S. Saify, M.Z. Khan, Zia-Ullah, M.I. Choudhary, Atta-ur-Rahman, S. Perveen, Z.H. Chohan, C.T. Supuran, Synthesis of coumarin derivatives with cytotoxic, antibacterial and antifungal activity. J. Enzyme Inhib. Med. Chem. 19, 373–379 (2004)
K.V. Sashidhara, J.N. Rosaiah, A. Kumar, G. Bhatia, A.K. Khanna, Synthesis of novel benzocoumarin derivatives as lipid lowering agents. Bioorg. Med. Chem. Lett. 20, 3065–3069 (2010)
I. Kostova, Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents 5, 29–46 (2005)
L.R. Zacharski, W.G. Henderson, F.R. Rickles, W.B. Forman, C. Cornell, A.J. Forcier, R. Edwards, E. Headley, S.H. Kim, J.F. O’Donnell, Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate: Final report of VA Cooperative Study # 75. Cancer 53, 2046–2052 (1984)
F.G. Medina, J.G. Marrero, M. Macías-Alonso, M.C. Gonzalez, I. Cordova-Guerrero, A.G.T. García, S. Osegueda-Robles, Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep. 32, 1472–1507 (2015)
S.J. Kashyap, V.K. Garg, P.K. Sharma, N. Kumar, R. Dudhe, J.K. Gupta, Thiazoles: having diverse biological activities. Med. Chem. Res. 21, 2123–2132 (2012)
N. Siddiqui, M.F. Arshad, W. Ahsan, M.S. Alam, Thiazoles: a valuable insight into the recent advances and biological activities. Int. J. Pharm. Sci. Drug Res. 1, 136–143 (2009)
S. Mirza, S.A. Naqvi, K.M. Khan, U. Salar, M.I. Choudhary, Facile synthesis of novel substituted aryl-thiazole (SAT) analogs via one pot multi-component reaction as potent cytotoxic agents against cancer cell lines. Bioorg. Chem. 70, 133–143 (2017)
M. Taha, N.H. Ismail, S. Imran, A. Wadood, F. Rahim, K.M. Khan, M. Riaz, Hybrid benzothiazole analogs as antiurease agent: Synthesis, and molecular docking studies. Bioorg. Chem. 69, 80–87 (2016)
M.I. Choudhary, A. Khan, K.M. Khan, N. Ambreen, Atia-tul-Wahab, Atta-ur-Rahman, Schiff bases of thiazoles: A new class of ureases inhibitors, US-Patent No. 9,447,057, Application No. 14/310,738, Patent Date: 09/20/2016.
U. Salar, M. Taha, K.M. Khan, N.H. Ismail, S. Imran, S. Perveen, S. Gul, A. Wadood, Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies. Eur. J. Med. Chem. 122, 196–204 (2016)
U. Salar, K.M. Khan, S. Chigurupati, S. Syed, S. Vijayabalan, M. Taha, A. Wadood, M. Riaz, M. Ghufran, S. Perveen, New hybrid scaffolds based on hydrazinyl thiazole substituted coumarin; as novel leads of dual potential; in vitro α-amylase inhibitory and antioxidant (DPPH and ABTS radical scavenging) activities. Med. Chem. 14, 86–101 (2018)
U. Salar, K.M. Khan, A. Jabeen, S. Hussain, A. Faheem, F. Naqvi, S. Perveen, Diversified thiazole substituted coumarins and chromones; as non-cytotoxic ROS and NO inhibitors. Lett. Drug Des. Dis. 17, 545–553 (2020)
K.M. Khan, S. Iqbal, M.A. Lodhi, G.M. Maharvi, Z. Ullah, M.I. Choudhary, Atta-ur-Rahman, S. Perveen, Bis-coumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg. Med. Chem. 12, 1963–1968 (2004)
U. Salar, A. Nizamani, F. Arshad, K.M. Khan, M.I. Fakhri, S. Perveen, N. Ahmed, M.I. Choudhary, Bis-coumarins; non-cytotoxic selective urease inhibitors and antiglycation agents. Bioorg. Chem. 91, 103170 (2019)
F. Chimenti, B. Bizzarri, A. Bolasco, D. Secci, P. Chimenti, A. Granese, S. Carradori, M. D’Ascenzio, M.M. Scaltrito, F. Sisto, Synthesis and anti-Helicobacter pylori activity of 4-(coumarin-3-yl)thiazol-2-ylhydrazone derivatives. J. Heterocyc. Chem. 47, 1269–1274 (2010)
B.C. Ding, H.J. Zhu, X.M. Li, F.F. Jia, B.L. Su, L.L. Zhang, Z.P. Cheng, J.Z. Yin, H. Zhong, Syntheses, ionic recognition and theoretical calculation of the fluorescence probe derived from coumarin. Jingxi Huagong 31, 695–698 (2014)
A. Gursoy, Coumarinylthiazolylhydrazone derivatives. J. Fac. Pharm. Istanbul Univ. 9, 77–84 (1973)
R.L. Lobo, B. Poojary, K. Manjunatha, N. Chidananda, V. Chandra, N.S. Kumari, Synthesis and evaluation of 4-aryl-2-[(2E)-2-substituted hydrazinyl]-1,3-thiazoles for possible antioxidant and antimicrobial activities. Pharm. Chem. 6, 61–69 (2014)
H. Osman, A. Arshad, C.K. Lam, M.C. Bagley, Microwave-assisted synthesis and antioxidant properties of hydrazinyl thiazolyl coumarin derivatives. Chem. Cent. J. 6, 32 (2012)
R.V. Rao, R.V. Kumar, A.V. Vardhan, A facile one-step synthesis of [3-(2-hydrazino-4-thiazolyl)coumarino]dimethylmethines and some 3-substituted 7H–6(6/8, 6,8-substituted-3-coumarino)-s-triazolo[3,4-b][1,3,4]thiadiazines. Phosphorus Sulfur Silicon Relat. Elem. 152, 257–264 (1999)
M. Yuan, W. Liu, Y. Zhang, H. Yan, D. Zhang, H. Liu, J. Wang, Synthesis and biological activity of N-[4-(coumarin-3-yl)thiazol-2-yl] aromatic aldehyde hydrazones under microwave irradiation. Chinese J. Org. Chem. 33, 1108–1112 (2013)
S. Benini, W.R. Rypniewski, K.S. Wilson, S. Miletti, S. Ciurli, S. Mangani, The complex of Bacillus pasteurii urease with acetohydroxamate anion from X-ray data at 1.55 Å resolution. JBIC 5, 110–118 (2000)
M. CCGI, (2016). Molecular Operating Environment (MOE), 2013.08. Chemical Computing Group Inc., Montreal.
Acknowledgements
The authors are thankful to the Pakistan Academy of Sciences for providing financial support to Project No. (5-9/PAS/440).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salar, U., Qureshi, B., Khan, K.M. et al. Aryl hydrazones linked thiazolyl coumarin hybrids as potential urease inhibitors. J IRAN CHEM SOC 19, 1221–1238 (2022). https://doi.org/10.1007/s13738-021-02377-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02377-8