Abstract
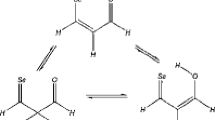
In this study, the molecular structure, tautomerization, conformational stability, and electronic energies of 25 enols and keto forms of methyl acetoacetate (MAA) were examined theoretically using DFT and experimentally by IR, Raman, and UV spectroscopy. According to the theoretical and experimental results, three more stable tautomer’s including an enol (2EU1-I) and two ketos (DK1 and DK2) forms in the gas phase, and solutions have been obtained. Using equilibrium ratios in the gas phase and different solvents at various levels of theory, the abundance of enol was determined. Outcomes indicated that the content of cis-enol or keto forms depended on to phase. In gas phase, cis-enol form is as major form. Conventional and unconventional intramolecular hydrogen bonds for cis-enol and keto forms, respectively, were considered via AIM. Also, using NBO in terms of E(2) values, the enol and keto forms, and the hydrogen bond strength interpreted. By recording the vibrational spectra in various polar and non-polar solvents, deuterated analog, deconvolution, and calculating potential energy distribution of cis-enol for MAA, the strong IR bands were obtained at about 1755–1715 cm−1 regions show the existence of two keto forms in the sample. Also, two medium intensity bands at 1654 and 1630 cm−1 were assigned to the chelated ring of cis-enol form. Finally, the UV spectra in different solvents and frontier molecular orbitals were shown that hydrogen bond strength in the enol form of MAA is weaker than AA. Also, the content of keto forms increased in polar solvents.







Similar content being viewed by others
References
O. Odunola, J. Woods, Synth. React. Inorg. Met.-Org. Chem. 31, 1297–1310 (2001)
S. Boughdiri, K. Essalah, J. Mol. Struct. Theochem. 958, 21–25 (2010)
M. Baumann, I.R. Baxendale, S.V. Ley, N. Nikbin, Beilstein J. Org. Chem. 7, 442–495 (2011)
X.-H. Zhang, Y.-H. Zhan, D. Chen, F. Wang, L.-Y. Wang, Dyes Pigm. 93, 1408–1415 (2012)
C.A. Bunton, F. Castañeda, J. Mol. Struct. 936, 132–136 (2009)
R.V. Ragavan, K.M. Kumar, V. Vijayakumar, et al. β-Keto esters from ketones and ethyl chloroformate: a rapid, general, efficient synthesis of pyrazolones and their antimicrobial, in silico and in vitro cytotoxicity studies. Org Med Chem Lett 3, 6 (2013)
V. Enchev, N. Gadjev, I. Angelov, S. Minkovska, A. Kurutos, N. Markova, T. Deligeorgiev, J. Mol. Struct. 1147, 380–387 (2017)
K. Vus, M. Girych, V. Trusova, G. Gorbenko, A. Kurutos, A. Vasilev, N. Gadjev, T. Deligeorgiev, J. Mol. Liq. 276, 541–552 (2019)
S.F. Tayyari, M. Zahedi-Tabrizi, H. Azizi-Toupkanloo, S.S. Hepperle, Y.A. Wang, Chem. Phys. 368, 62–65 (2010)
V. Darugar, M. Vakili, S.F. Tayyari, P.E. Hansen, F.S. Kamounah, J. Mol. Liq. 334, 116035 (2021)
S.F. Tayyari, S. Salemi, M.Z. Tabrizi, M. Behforouz, J. Mol. Struct. 694, 91–104 (2004)
N.V. Belova, V.V. Sliznev, H. Oberhammer, G.V. Girichev, J. Mol. Struct. 978, 282–293 (2010)
S.F. Tayyari, F. Naghavi, S. Pojhan, R.W. McClurg, R.E. Sammelson, J. Mol. Struct. 987, 241–254 (2011)
S.F. Tayyari, B. Chahkandi, S. Mehrani, R.W. McClurg, C.A. Keyes, R.E. Sammelson, J. Mol. Struct. 1015, 74–85 (2012)
K.T. Smith, S.C. Young, J.W. DeBlasio, C.S. Hamann, J. Chem. Educ. 93, 790–794 (2016)
M.M. Folkendt, B.E. Weiss-Lopez, J.P. Chauvel Jr., N.S. True, J. Phys. Chem. 89, 3347–3352 (1985)
J.L. Burdett, M.T. Rogers, J. Am. Chem. Soc. 86, 2105–2109 (1964)
N.V. Belova, H. Oberhammer, G.V. Girichev, J. Phys. Chem. A 108, 3593–3597 (2004)
D. Berthomieu, J. Tortajada, J.P. Morizur, H.E. Audier, Org. Mass Spectrom. 26, 601–607 (1991)
M.M. Schiavoni, H.E. Di Loreto, A. Hermann, H.-G. Mack, S.E. Ulic, C.O. Della Védova, Keto–enol tautomerism in β-ketoesters: CH3C(O)CHXC(O)OY (X = H, Cl; Y = CH3, C2H5). Vibrational analyses, NMR spectra and quantum chemical calculations. J. Raman Spectrosc. 32, 319–329 (2001)
Gaussian09, Revision A. “1, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, v. Barone, b. Mennucci, ga petersson et al., gaussian.” Inc., Wallingford CT 121, 150–166 (2009)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
A.D. Becke, J. Chem. Phys. 98, 5648–5652 (1993)
M. Head-Gordon, J.A. Pople, M.J. Frisch, Chem. Phys. Lett. 153, 503–506 (1988)
S. Grimme, J. Chem. Phys. 124, 034108 (2006)
S.M. Aguilera-Segura, J.M. Seminario, J. Phys. Chem. 118, 1397–1406 (2014)
J. Tomasi, M. Persico, Chem. Rev. 94, 2027–2094 (1994)
B. Mennucci, J. Tomasi, J. Chem. Phys. 106, 5151–5158 (1997)
A. Bondi, J. Phys. Chem. 68, 441–451 (1964)
F. Dolati, S.F. Tayyari, M. Vakili, J. Mol. Struct. 1094, 264–273 (2015)
M. Gholamhoseinpour, S.F. Tayyari, S. Emamian, Can. J. Chem. 94, 818–826 (2016)
H. Fakheri, S.F. Tayyari, M. Momen Heravi, A. Morsali, Egypt. J. Chem. 64(2), 2–7 (2021)
R. Dennington, T. Keith, J. Millam, GaussView (Semichem Inc., Shawnee Mission, KS, 2009)
R.F. Bader, A quantum theory. (Clarendon, Oxford, UK, 1990)
F. Biegler-König, Calculation of atomic integration data. J. Comput. Chem. 21, 1040–1048 (2000)
A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899–926 (1988)
K.B. Wiberg, Tetrahedron 24, 1083–1096 (1968)
A.E. Reed, R.B. Weinstock, F. Weinhold, J. Chem. Phys. 83, 735–746 (1985)
P. Klaeboe, A. Horn, C.J. Nielsen, V. Aleksa, G.A. Guirgis, J.K. Wyatt, H.W. Dukes, J. Mol. Struct. 1034, 207–215 (2013)
A. Kurutos, Y. Shindo, Y. Hiruta, K. Oka, D. Citterio, Dyes and Pigments, 181, p. 108611 (2020)
G. Schreckenbach, T. Ziegler, J. Phys. Chem. 99, 606–611 (1995)
G. Rauhut, S. Puyear, K. Wolinski, P. Pulay, J. Phys. Chem. 100, 6310–6316 (1996)
V. Rarh, Organic Compounds containing Nitrogen (2008)
V. Bertolasi, P. Gilli, V. Ferretti, G. Gilli, J. Am. Chem. Soc. 113, 4917–4925 (1991)
E. Espinosa, E. Molins, C. Lecomte, Chem. Phys. Lett. 285, 170–173 (1998)
S.F. Tayyari, F. Milani-Nejad, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 56, 2679–2691 (2000)
S. Mehrani, et al., Vibrational spectra, normal coordinate analysis, and structure of keto form of acetylacetone: a DFT approach. Egypt. J. Chem. 63(4), 1149–1159 (2020)
T. Yamaji, T. Saito, K. Hayamizu, M. Yanagisawa, O. Yamamoto, N. Wasada, K. Someno, S. Kinugasa, K. Tanabe, T. Tamura, SDBS, NMR, Natl. Inst. Adv. Ind. Sci. Technol. (AIST), Japan (2014)
V. Darugar, M. Vakili, S.F. Tayyari, F.S. Kamounah, J. Mol. Graph. Model. 107, 107976 (2021)
A. Kurutos, I. Orehovec, D. Saftić, L. Horvat, I. Crnolatac, I. Piantanida, T. Deligeorgiev, Dyes Pigm. 158, 517–525 (2018)
N. Nagashima, S. Kudoh, M. Takayanagi, M. Nakata, J. Phys. Chem. A 105, 10832–10838 (2001)
S. Coussan, C. Manca, Y. Ferro, P. Roubin, Chem. Phys. Lett. 370, 118–125 (2003)
L. Messaadia, G. El Dib, A. Ferhati, A. Chakir, Chem. Phys. Lett. 626, 73–79 (2015)
S. Coussan, Y. Ferro, A. Trivella, M. Rajzmann, P. Roubin, R. Wieczorek, C. Manca, P. Piecuch, K. Kowalski, M. Włoch, J. Phys. Chem. A 110, 3920–3926 (2006)
Acknowledgements
The authors of this work appreciate the financial support of the Ferdowsi University of Mashhad, Iran (Grant No. 3/45820).
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Ms. Mozhgan Mahmoudi Aval involved in methodology, formal analysis and investigation, and writing the original draft. Dr. ANP participated in conceptualization, supervision, writing-review and editing. Dr. MV participated in conceptualization, supervision, and writing-review and editing. Prof. Syed Faramarz Tayyari involved in supervision, writing-review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahmoudi Aval, M., Nakhaei Pour, A., Vakili, M. et al. Conformational analysis, tautomerization, and vibrational spectra of methyl acetoacetate. J IRAN CHEM SOC 19, 1081–1094 (2022). https://doi.org/10.1007/s13738-021-02362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02362-1