Abstract
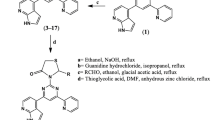
1,3-Thiazolidin-4-one derivatives bearing indole and bi-pyrimidine nucleuses (1–12) was designed and analyzed computationally for bioactivity. The synthesized compounds were characterized using FTIR, NMR and Mass Spectroscopy. The compounds (1–12) were then assessed for antibacterial therapeutic effects against the microbes [S. aureus (ATCC-25923), S. epidermidis (ATCC-29887),E. coli (ATCC-25922), and P. mirabilis (ATCC-25933)] using disk diffusion methods. The percent viability of the cells was analyzed by MTT against HepG2 cells to observe the cytotoxicity. Additionally, the molecular docking was carried out for the compounds (1–12) against the receptor GlcN-6P to assess the extent of H-bonding and the binding affinities.
Graphic abstract




Similar content being viewed by others
References
S.N. Dighe, T.A. Collet, Eur. J. Med. Chem. 112326, 199 (2020)
R. Zanni, M. Galvez-Llompart, J. Machuca, R. Garcia-Domenech, E. Recacha, A. Pascual, J.M. Rodriguez-Martinez, J. Galvez, Eur. J. Med. Chem. 233, 137 (2017)
M.L. Fascio, M.I. Errea, N.B. D’Accorso, Eur. J. Med. Chem. 666, 90 (2015)
Y. Xue, X. He, T. Yang, Y. Wang, Z. Liu, G. Zhang, Y. Wang, K. Wang, L. Zhang, L. Zhang, Eur. J. Med. Chem. 111618, 182 (2019)
V. Venepally, R. Chandra, R. Jala, Eur. J. Med. Chem. 113, 141 (2017)
Z.-X. He, T.-Q. Zhao, Y.-P. Gong, X. Zhang, L.-Y. Ma, H.-M. Liu, Eur. J. Med. Chem. 112458, 200 (2020)
S. Kumar, B. Narasimhan, Chem. Cent. J. 38, 12 (2018)
M. Arshad, J. Iran Chem. Soc. 1305, 17 (2020)
M. Arshad, D. Ahmad, Chem. Data Collect. 100405, 28 (2020)
N.K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C.H. Kim, A.K. Verma, E.H. Choi, Molecules 6620, 18 (2013)
S.-Y. Wang, X.-C. Shi, P. Laborda, Eur. J. Med. Chem. 111847, 185 (2020)
E. Yamuna, R.A. Kumar, M. Zeller, K.J.R. Prasad, Eur. J. Med. Chem. 228, 47 (2012)
S. Dadashpour, S. Emami, Eur. J. Med. Chem. 9, 150 (2018)
S. Carradori, B. Bizzarri, M. D’Ascenzio, C.D. Monte, R. Grande, D. Rivanera, A. Zicari, E. Mari, M. Sabatino, A. Patsilinakos, R. Ragno, D. Secci, Eur. J. Med. Chem. 274, 140 (2017)
S. Koppireddi, J.R. Komsani, S. Avula, S. Pombala, S. Vasamsetti, S. Kotamraju, R. Yadla, Eur. J. Med. Chem. 305, 66 (2013)
D. S. daSilva, C. E. H. daSilva, M. S. P. Soares, J. H. Azambuja, T. R. deCarvalho, G. C. Zimmer, C. P. Frizzo, E. Braganhol, R. M. Spanevello, W. Cunico, Eur. J. Med. Chem. 574, 124 (2016).
D. Secci, S. Carradori, B. Bizzarri, P. Chimenti, C.D. Monte, A. Mollica, D. Rivanera, A. Zicari, E. Mari, G. Zengin, A. Aktumsek, Eur. J. Med. Chem. 144, 117 (2016)
C.D. Monte, S. Carradori, B. Bizzarri, A. Bolasco, F. Caprara, A. Mollica, D. Rivanera, E. Mari, A. Zicari, A. Akdemir, D. Secci, Eur. J. Med. Chem. 82, 107 (2016)
R.K. Rawal, R. Tripathi, S.B. Katti, C. Pannecouque, E.D. Clercq, Eur. J. Med. Chem. 2800, 43 (2008)
S. Raza, S.P. Srivastava, D.S. Srivastava, A.K. Srivastava, W. Haq, S.B. Katti, Eur. J. Med. Chem. 611, 63 (2013)
M. D’Ascenzio, B. Bizzarri, C.D. Monte, S. Carradori, A. Bolasco, D. Secci, D. Rivanera, N. Faulhaber, C. Bordón, L. Jones-Brando, Eur. J. Med. Chem. 17, 86 (2014)
G. Revelant, S. Huber-Villaume, S. Dunand, G. Kirsch, H. Schohn, S. Hesse, Eur. J. Med. Chem. 102, 94 (2015)
Ł. Popiołek, A. Biernasiuk, A. Malm, Phosphorus, Sulfur, and Silicon and the Related Elements, 190 (2015).
I. Piątkowska-Chmiel, Farmacia. 258, 67 (2019)
A.D. Taranalli, A.R. Bhat, S. Srinivas, E. Saravanan, Indian J. Pharm Sci. 159, 70 (2008)
M.F. Ansari, S.M. Siddiqui, K. Ahmad, F. Avecilla, S. Dharavath, S. Gourinath, A. Azam, Eur. J. Med. Chem. 393, 124 (2016)
W. Cunico, C.R.B. Gomes, M.L.G. de Ferreira, L.R. Capri, M.R.L. Santos, P.M. Sa, N. Boechat, M.M. Bastos, L.C. Maciel, L.M.U. Mayer, Lett. Org. Chem. 505, 4 (2007)
B. Qi, Y. Yang, H. He, X. Yue, Y. Zhou, X. Zhou, Y. Chen, M. Liu, A. Zhang, F. Wei, Eur. J. Med. Chem. 368, 146 (2018)
A. Zarghi, L. Najafnia, B. Daraee, O.G. Dadrass, M. Hedayati, Bioorg. Med. Chem. Lett. 5634, 17 (2007)
D. Shahabi, H. Tavakol, Appl. Surf. Sci. 267, 420 (2017)
H. Tavakol, Mol. Simul. 391, 36 (2010)
M. Arshad, M.S. Khan, S.A.A. Nami, D. Ahmad, Russ. J. Gen. Chem. 2154, 88 (2018)
M. Arshad, M. Shoeb Khan, S.A.A. Nami, D. Ahmad, Russ. J. Gen. Chem. 2154, 88 (2018)
M. Arshad, Int. J. Pharm. Sci. Res. 12, 9 (2018)
E.A. Alodeani, M. Arshad, M.A. Izhari, Eur. J. Pharm. Med. Res. 296, 2 (2015)
E.A. Alodeani, M. Arshad, M.A. Izhari, Asian Pac J. Health Sci. 41, 2 (2015)
E.A. Alodeani, M. Arshad, M.A. Izhari, Eur. J. Pharm. Med. Res. 324, 2 (2015)
A.R. Bhat, M. Arshad, E.J. Lee, S. Pokharel, I. Choi, F. Athar, Chem. Biod. 2267, 10 (2013)
E.A. Alodeani, M. Arshad, M.A. Izhari, Eur. J. Pharm. Biomed. Sci. 504, 1 (2014)
M. Arshad, A.R. Bhat, K.K. Hoi, I. Choi, F. Athar, Chin. Chem. Lett. 1559, 28 (2017)
M. Arshad, Int. J. Pharm. Pharmaceut. Sci. 16, 9 (2014)
M. Arshad, Int. J. Pharm. Sci. Res. 149, 5 (2014)
M. Arshad, Int. J. Pharm. Sci. Res. 1000, 5 (2014)
M. Arshad, T.A. Khan, Int. J. Pharm. Sci. Res. 149, 5 (2014)
M.A. Tazeem, A.R. Bhat, F. Athar, J. Nat. Sci. Biol. Med. 2, 1–156 (2011)
M.A. Tazeem, A.R. Bhat, F. Athar, Journal of Natural Science. Biology and Medicine 1–156, 2 (2011)
M. Arshad, Int. J. Pharm. Sci. Res. 35, 9 (2018)
M. Arshad, M.S. Khan, S.A. AsgharNami, D. Ahmad, Russ J Gen Chem. 2154, 88 (2018)
M. Arshad, Russ. J Gen Chem. 1886, 88 (2018)
M. Arshad, Eur. J. Pharm. Med. Res. 511, 4 (2017)
E.A. Alodeani, M. Arshad, M.A. Izhari, Asian Pac J. Trop Biomed. 676, 5 (2015)
E.A. Alodeani, M. Arshad, M.A. Izhari, American Journal of Pharm Tech research 150, 5 (2015)
M. Arshad, S.N. Appl, Sci. 467, 2 (2020)
M.K. Gupta, T.V. Neelakantan, M. Sanghamitra, R.K. Tyagi, A. Dinda, S. Maulik, C.K. Mukhopadhyay, S.K. Goswami, Antioxid. Redox Signal. 1081, 8 (2006)
T. Mosmann, J. Immunol. Methods 55, 65 (1983)
M. Arshad, A.R. Bhat, S. Pokharel, E.J. Lee, F. Athar, I. Choi, Eur. J. Med. Chem. 229, 71 (2014)
M. Arshad, M.S. Khan, S.A.A. Nami, Russ J Gen Chem. 1851, 89 (2019)
M. Arshad, M. Shadab, Eur. J. Pharm. Med. Res. 364, 4 (2017)
M. Arshad, M. Shadab, Eur. J. Pharm. Med. Res. 447, 4 (2017)
P.S. Nayab, R. Arif, M. Arshad, Rahisuddin. Heterocyclic letters 223, 5 (2015)
Acknowledgements
Dr. Mohammad Arshad is highly thankful to Dr. Feras Al-Marshad, The Dean College of Medicine, Al-Dawadmi, Shaqra University Kingdom of Saudi Arabia, for his kind support to accomplish this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Arshad, M., Khan, M.S. & Nami, S.A.A. Synthesis, characterization, biological and molecular docking assessment of computationally bioactive 1,3-thiazolidin-4-one derivatives bearing indole and bi-pyrimidine moieties. J IRAN CHEM SOC 18, 2397–2406 (2021). https://doi.org/10.1007/s13738-021-02200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02200-4