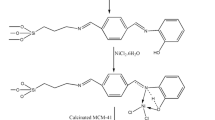
Abstract
In this work, the surface of mesoporous MCM-41 was modified with guanidine, and then, Nickel particles have become immobilized on its surface (Ni-guanidine@MCM-41NPs). This heterogeneous catalyst has been identified by various techniques including: low-angle X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, inductively coupled plasma, thermal gravimetric analysis and N2 adsorption–desorption measurement isotherms, and its catalytic application was studied in the synthesis of 4,4ʹ-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ol) derivatives and symmetric di-aryl sulfides. The prepared organometallic complex could be isolated, post-reaction, by simple filtration for several consecutive cycles without a notable change in its catalytic activity.









Similar content being viewed by others
References
C.M. Marson, Chem. Soc. Rev. 41, 7712 (2012)
B. Jiang, T. Rajale, W. Wever, S.J. Tu, G. Li, Chem. Asian J. 5, 2318 (2010)
A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
H. Wu, Y. Gou, J. Wang, L. Tao, Macromol. Rapid Commun. 39, 1800064 (2018)
D. Das, ChemistrySelect 1, 1959 (2016)
C. Shen, X.F. Wu, Chem. Eur. J. 23, 2973 (2017)
R.N. Mahajan, F.H. Havaldar, P.S. Fernandes, J. Indian Chem. Soc. 68, 245 (1991)
P.M.S. Chauhan, S. Singh, R.K. Chatterjee, ChemInform 24, 72 (1993)
M. Keshavarz, M. Vafaei-Nezhad, Catal. Lett. 146, 353 (2016)
M. Baghernejad, K. Niknam, Int. J. Chem. 4, 52 (2012)
C.S. Yao, C.X. Yu, S.J. Tu, D.Q. Shi, X.S. Wang, Y.Q. Zhu, H.Z. Yang, J. Fluor. Chem. 128, 105 (2007)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh, A. Hasaninejad, Appl. Catal. A Gen. 467, 61 (2013)
M.N. Elinson, A.S. Dorofeev, R.F. Nasybullin, G.I. Nikishin, Synthesis (Stuttg) 2008, 1933 (2008)
K. Niknam, D. Saberi, M. Sadegheyan, A. Deris, Tetrahedron Lett. 51, 692 (2010)
E. Soleimani, S. Ghorbani, M. Taran, A. Sarvary, Comptes Rendus Chim. 15, 955 (2012)
A. Hasaninejad, M. Shekouhy, A. Zare, S.M.S.H. Ghattali, N. Golzar, J. Iran. Chem. Soc. 8, 411 (2011)
K. Niknam, M.S. Habibabad, A. Deris, N. Aeinjamshid, Chem. Mon. 144, 987 (2013)
K. Niknam, S. Mirzaee, Synth. Commun. 41, 2403 (2011)
Y. Zhang, K.C. Ngeow, J.Y. Ying, Org. Lett. 9, 3495 (2007)
I.P. Beletskaya, V.P. Ananikov, Chem. Rev. 111, 1596 (2011)
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
L. Rout, T.K. Sen, T. Punniyamurthy, Angew. Chem. Int. Ed. 46, 5583 (2007)
M. Moorthy, A. Govindaraj, B. Madheswaran, B. Kannan, R. Rangappan, ChemistrySelect 1, 4833 (2016)
D. Giuliani, A. Ottani, D. Zaffe, M. Galantucci, F. Strinati, R. Lodi, S. Guarini, Neurobiol. Learn. Mem. 104, 82 (2013)
L. Argueta-Figueroa, O. Martínez-Alvarez, J. Santos-Cruz, R. Garcia-Contreras, L.S. Acosta-Torres, J. de la Fuente-Hernández, M.C. Arenas-Arrocena, Mater. Sci. Eng. C 76, 1305 (2017)
V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010)
A. Ghorbani-Choghamarani, M. Mohammadi, Z. Taherinia, J. Iran. Chem. Soc. 16, 411 (2019)
M. Nikoorazm, A. Ghorbani-Choghamaranai, M. Khanmoradi, P. Moradi, J. Porous Mater. 25, 1831 (2018)
H. Filian, A. Ghorbani-Choghamarani, E. Tahanpesar, J. Porous Mater. (2018) (in press)
N. Gutta, V.K. Velisoju, A. Chatla, V. Boosa, J. Tardio, J. Patel, V. Akula, Energy Fuels 32, 4008 (2018)
G. Maria, A.I. Stoica, I. Luta, D. Stirbet, G.L. Radu, Microporous Mesoporous Mater. 162, 80 (2012)
A. Benhamou, J.P. Basly, M. Baudu, Z. Derriche, R. Hamacha, J. Colloid Interface Sci. 404, 135 (2013)
A.G. Thomé, F. Schroeter, P. Bottke, J. Wittayakun, F. Roessner, Microporous Mesoporous Mater. 274, 342 (2019)
S.A. Idris, S.R. Harvey, L.T. Gibson, J. Hazard. Mater. 193, 171 (2011)
Y. Sun, X.W. Liu, W. Su, Y. Zhou, L. Zhou, Appl. Surf. Sci. 253, 5650 (2007)
M. Abdollahi-Alibeik, M. Pouriayevali, Catal. Commun. 22, 13 (2012)
X. Wang, G. Wu, J. Li, N. Zhao, W. Wei, Y. Sun, J. Mol. Catal. A: Chem. 276, 86 (2007)
Y. Huang, W. Hao, G. Ding, M.Z. Cai, J. Organomet. Chem. 715, 141 (2012)
B. Karami, M. Farahi, S. Akrami, D. Elhamifar, New J. Chem. 42, 12811 (2018)
M. Eslami, M.G. Dekamin, L. Motlagh, A. Maleki, Green Chem. Lett. Rev. 11, 36 (2018)
J.N. Appaturi, M. Selvaraj, S.B. Abdul Hamid, M.R. Bin Johan, Microporous Mesoporous Mater. 260, 260 (2018)
T. Tamoradi, M. Ghadermazi, A. Ghorbani-Choghamarani, Catal. Lett. 148, 857 (2018)
A. Ghorbani-Choghamarani, Z. Seydyosefi, B. Tahmasbi, Appl. Organomet. Chem. 32, e4396 (2018)
Acknowledgements
This work was supported by the research facilities of Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filian, H., Ghorbani-Choghamarani, A. & Tahanpesar, E. Ni-guanidine@MCM-41 NPs: a new catalyst for the synthesis of 4,4ʹ-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) and symmetric di-aryl sulfides. J IRAN CHEM SOC 16, 2673–2681 (2019). https://doi.org/10.1007/s13738-019-01727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01727-x