Abstract
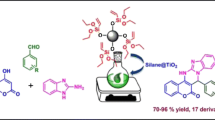
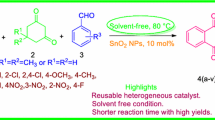
New highly efficient method for safe, green and facile synthesis of spirooxindole derivatives have been presented. SnO2 nanoparticles (SnO2 NPs) as effective catalyst were synthesized through chemical precipitation method and characterized in details using FTIR, XRD, SEM and EDS methods. Obtained nano tin oxide particles were used as heterogeneous catalyst for the one-pot synthesis of spirooxindoles at room temperature. High–excellent yields of products, short reaction times, room temperature conditions and reusability of catalyst are the main advantages of this method. Evaluation of antimicrobial activity of some synthesized compounds were performed against eight bacteria. Agar diffusion method was used for determining preliminary antibacterial activities and to assess the bacterio-static activity of compounds with inhibition zone, micro-well dilution assay method was used. Obtained results showed that (5j) was active against all tested microorganisms and was the most effective compound.






Similar content being viewed by others
References
K. Azizi, M. Karimi, H.R. Shaterian, A. Heydari, RSC Adv. 4, 42220 (2014)
M. Nasrollahzadeh, S.M. Sajadi, A. Rostami-Vartooni, S. Mamand Hussin, J. Colloid Interface Sci. 459, 183 (2015)
M. Mujahid Alam, S. Merajuddin Ahmed, A. Imtiaz Ansari, Int. J. Basic Appl. Chem. Sci. 4, 24 (2014)
S.M. Vahdat, R.S. Ghafouri, S. Baghery, J. Chem. Sci. 33, 579 (2014)
R. Ranjbar Karimi, S. Hashemi-Uderji, A. Bazmandegan-Shamili, Chin. J. Chem. 29, 1624 (2011)
N. Shahrivar Fallah, M. Mokhtary, J. Taibah Univ. Sci. 9, 531 (2015)
Z. Peng, Z. Shi, M. Liu, Chem. Commun. 29, 2125 (2000)
J.A. Ayllon, M.L. Cantu, Appl. Phys. A Mater. Sci. Process. 95, 249 (2008)
B.Y. Wei, M.C. Hsub, P.G. Su, H.M. Lin, R.J. Wu, H.J. Lai, Sens. Actuators B 101, 81 (2004)
Y.L. Liu, H.F. Yang, Y. Yang, Z.M. Liu, G.L. Shen, R.Q. Yu, Thin Solid Films 497, 355 (2006)
H.Y. Jang, H. Lee, S.I. Mho, J. Korean Phys. Soc. 53, 3588 (2008)
A.Y. Jogalekar, R.G. Jaiswal, R.V. Jayaram, J. Chem. Technol. Biotechnol. 71, 234 (1998)
A.A. Yelwande, M.E. Navgire, D.T. Tayde, B.R. Arbad, M.K. Lande, Bull. Korean Chem. Soc. 33, 1856 (2012)
S. Pal, M. Nasim Khan, S. Karamthulla, L.H. Choudhury, Tetrahedron Lett. 56, 359 (2015)
D. Cheng, Y. Ishihara, B. Tan, C.F. Barbas, ACS Catal. 4, 743 (2014)
M.M.M. Santos, Tetrahedron 70, 9735 (2014)
D. Ke, L. Yipin, N.C. Zaneta, W. Guoping, Q. Sanjeev, S. Su, G. Wei, Q. Dongguang, S. Jeanne, K. Krzysztof, J. Med. Chem. 49, 3432 (2006)
N.R. Ball-Jones, J.J. Badillo, A.K. Franz, Org. Biomol. Chem. 10, 5165 (2012)
Y.B. Wagh, Y.A. Tayade, S.A. Padvi, B.S. Patil, N.B. Patil, D.S.A. Dalal, Chin. Chem. Lett. 26, 1273 (2015)
S.A. Padvi, Y.A. Tayade, Y.B. Wagh, D.S. Dalal, Chin. Chem. Lett. 27, 714 (2016)
M. Dabiri, Z. Noroozi Tisseh, M. Bahramnejad, A. Bazgir, Ultrason. Sonochem. 18, 1153 (2011)
A. Hasaninejad, N. Golzar, M. Beyrati, A. Zare, M.M. Doroodmand, J. Mol. Catal. A Chem. 372, 137 (2013)
C. Wu, R. Shen, J. Chen, C. Hu, Bull. Korean Chem. Soc. 34, 2431 (2013)
R.Y. Guo, Z.M. An, L.P. Mo, S.T. Yang, H.X. Liu, S.X. Wang, Z.H. Zhang, Tetrahedron 69, 9931 (2013)
B. Maheshwar Rao, G. Niranjan Reddy, T. Vijaikumar Reddy, B.L.A. Prabhavathi Devi, R.B.N. Prasad, J.S. Yadav, B.V. Subba Reddy, Tetrahedron Lett. 54, 2466 (2013)
M. Srivastava, P. Rai, J. Singh, J. Singh, RSC Adv. 4, 30592 (2014)
P. Rai, M. Srivastava, J. Singh, J. Singh, New J. Chem. 38, 3181 (2014)
S. Riyaz, A. Indrasena, A. Naidu, P.K. Dubey, Indian J. Chem. Sect. B 53, 1442 (2014)
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS Comb. Sci. 14, 38 (2012)
H.R. Shaterian, M. Arman, F. Rigi, J. Mol. Liq. 158, 145 (2011)
M.N. Elinson, A.S. Dorofeev, F.M. Miloserdov, G.I. Nikishin, Mol. Divers. 13, 47 (2009)
K. Rad-Moghadam, L. Youseftabar-Miri, Tetrahedron 67, 5693 (2011)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
R.-Y. Guo, P. Wang, G.D. Wang, L.P. Mo, Z.H. Zhang, Tetrahedron 69, 2056 (2013)
X.N. Zhang, Y.X. Li, Z.H. Zhang, Tetrahedron 67, 7426 (2011)
S.M. Sadeghzadeh, M.A. Nasseri, Catal. Today 217, 80 (2013)
H. Naeimi, Z. Rashid, A.H. Zarnani, R. Ghahremanzadeh, New J. Chem. 3, 348 (2014)
B. Chandan, K. Ashis, P. Animesh, RSC Adv. 5, 85202 (2015)
L. Moradi, K. Rabiei, F. Belali, Synth. Commun. 46, 1283 (2016)
L. Moradi, M. Tadayon, J. Saudi Chem. Soc. (2017). https://doi.org/10.1016/j.jscs.2017.07.004
L. Moradi, G.R. Najafi, H. Saeidiroshan, Iran J. Catal. 5, 357 (2015)
A.N. Naje, A.S. Norry, A.M. Suhail, Int. J. Innov. Res. Sci. Eng. Technol. 2, 7068 (2013)
M. Golestanzadeh, H. Naeimi, Z. Zahraie, Chem. Sel. 1, 6490 (2016)
M. Hosseini-Sarvari, M. Tavakolian, Comb. Chem. High Throughput Screen. 15, 826 (2012)
M. Dabiri, M. Bahramnejad, M. Baghbanzadeh, Tetrahedron 65, 9443 (2009)
D.S. Raghuvanshi, K.N.J. Singh, J. Heterocycl. Chem. 47, 1323 (2010)
S.L. Zhu, S.J. Ji, Y. Zhang, Tetrahedron 63, 9365 (2007)
S. Javanshir, N. Saghiran Pourshiri, Z. Dolatkhah, M. Farhadnia, Monatsh. Chem. 148, 703 (2017)
R. Jamatia, A. Gupta, A.K. Pal, RSC Adv. 6, 20994 (2016)
A.F. Mahmoud, F.F. Abd El-Latif, A.M. Ahmed, Chin. J. Chem. 28, 91 (2010)
D.R. Chandam, A.G. Mulik, D.R. Patil, M.B. Deshmukh, Res. Chem. Intermed. 42, 1411 (2016)
K. Bikash, N. Anupam, B.A. Julie, Tetrahedron Lett. 53, 5004 (2012)
J. Davarpanah, A.R. Kiasat, RSC Adv. 4, 4403 (2014)
F. Mohamadpour, M.T. Maghsoodlou, R. Heydari, M. Lashkari, Res. Chem. Intermed. 42, 7841 (2016)
D.N. Survase, H.V. Chavan, S.B. Dongare, V.B. Helavi, Synth. Commun. 46, 1665 (2016)
R. Yufeng, Y. Bo, L. Xiali, Catal. Sci. Technol. 6, 4283 (2016)
A. Khalafi-Nezhad, E.S. Shahidzadeh, S. Sarikhani, F. Panahi, J. Mol. Catal. A Chem. 379, 1 (2013)
M. Keshavarz, J. Iran. Chem. Soc. 13, 553 (2016)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, A. Shirinfeshan, Green Chem. 14, 1696 (2012)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 5004 (2012)
H. Dolati, A. Habibi, S.A. Ayatollahi, S.M. Mahdavi, Y. Valizadeh, J. Chem. Soc. Pak. 38, 517 (2016)
Acknowledgements
We gratefully acknowledge the Chemistry Department of University of Kashan (Grant no. 784946) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moradi, L., Ataei, Z. & Zahraei, Z. Convenient synthesis of spirooxindoles using SnO2 nanoparticles as effective reusable catalyst at room temperature and study of their in vitro antimicrobial activity. J IRAN CHEM SOC 16, 1273–1281 (2019). https://doi.org/10.1007/s13738-019-01598-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01598-2