Abstract
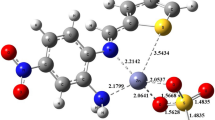
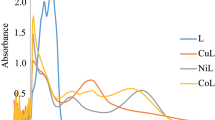
Synthesis of two homoleptic and five heteroleptic zinc (II) carboxylate complexes with two different ligands: HL1 = 3-chlorobenzoic acid and HL2 = 2-(4-chlorophenyl) acetic acid have been reported in this paper. The general formulae of the two homoleptic complexes are: Zn(L1)2(1), and Zn(L2)2(2). Similarly, the general formulae for two different types of heteroleptic complexes are: (Type-I): [(Zn)2(L1)4(bipy)2(H2O)] (3), [Zn(L2)2(bipy)(H2O)] (4) and (Type-II): [ZnL1L2] (5), [ZnL1L2(py)] (6) and [ZnL1L2(bipy)] (7). The synthesized complexes were characterized in the solid state by FT-IR, CHN analyses and in solution state by NMR (1H, 13C) spectroscopy. Complexes 3 and 4 were also characterized by single crystal analysis where data revealed that the geometry around each Zn atom is distorted trigonal bipyramidal and octahedral, respectively. The synthesized complexes were interacted with SS-DNA and CTAB (surfactant). Interaction of the synthesized compounds with SS-DNA was studied by UV–visible spectroscopy and viscometry and intercalative mode of interaction was exhibited. Moreover, the interaction of the synthesized complexes with CTAB was studied by conductometry showing a strong binding that is evident from higher CMC and negative Gibbs free energy of micellization (ΔGm) values. The tested compounds were further screened for in vitro antibacterial and antifungal activities and were found active against the studied strains of bacteria and fungus.











Similar content being viewed by others
References
J.G. Zeikus, M.K. Jain, P. Elankovan, Appl. Microbiol. Biotechnol. 51, 545 (1999)
M. Sirajuddin, S. Ali, V. McKee, H. Ullah, Spectrochim. Acta Part A 138, 569 (2015)
S. Caglar, S. Demir, Z. Heren, O. Büyükgüngör, Polyhedron 30, 1389 (2011)
L. Pellerito, L. Nagy, Coord. Chem. Rev. 224, 111 (2002)
A. Bianco, N. Uccella, Food Res. Int. 33, 475 (2000)
Y. Kim, J.Y. Cho, J.H. Kuk, J.H. Moon, J.I. Cho, Y.C. Kim, K.H. Park, Curr. Microbiol. 48, 312 (2004)
R. Cini, Comment. Inorg. Chem. 22, 151 (2000)
H. Yin, S.X. Liu, J. Mol. Struct. 918, 165 (2009)
J. Zhao, X. Shi, G. Li, X. Wang, C. Li, Q. Yang, Inorg. Chim. Acta 383, 185 (2012)
D.W. Christianson, J.D. Cox, Ann. Rev. Biochem. 68, 33 (1999)
D. Dey, S. Roy, R.N.D. Purkayastha, R. Pallepogu, P. McArdle, J. Mol. Struct. 1053, 127 (2013)
J.X. Chen, W.E. Lin, M. Chen, F.C. Que, L. Tao, X.L. Cen, Y.M. Zhou, W.H. Chen, Inorg. Chim. Acta 409, 195 (2014)
N. Muhammad, M. Ikram, A. Wadood, S. Rehman, S. Shujah, M. Erum, S. Ghufran, M. Rahim, C. Shah, Schulzke, Spectrochim. Acta Part A 190, 368 (2018)
D.K. Mishra, U.K. Singha, A. Das, S. Dutta, P. Kar, A. Chakraborty, A. Sen, B. Sinha, J. Coord. Chem. https://doi.org/10.1080/00958972.2018.1476687(2018)
M. Sirajuddin, S. Ali, A. Badshah, J. Photochem. Photobio. B 124, 1 (2013)
R.C. Maurya, J. Chourasia, P. Sharma, Ind. J. Chem. A 46, 1594 (2007)
I. Kani, J. Chem. Crystallogr. 42, 832 (2012)
Bruker, APEX2, SAINT and SADABS (Bruker AXS Inc., Madison, 2008)
G.M. Sheldrick, Acta Cryst. A71, 3 (2015)
G.M. Sheldrick, Acta Cryst. C71, 3 (2015)
A. Altomare, G. Cascarano, C. Giacovazzo, A. Gualardi, J. Appl. Crystallogr. 26, 343 (1993)
Z. Moghadam, K. Akhbari, J. White, A. Phuruangrat, Polyhedron 153, 286 (2018)
A. Abbas, S. Murtaza, M.N. Tahir, S. Shamim, M. Sirajuddin, U.A. Rana, K. Naseem, H. Rafique, J. Mol. Struct. 1117, 269 (2016)
S. Murtaza, S. Shamim, N. Kousar, M.N. Tahir, M. Sirajuddin, U.A. Rana, J. Mol. Struct. 1107, 99 (2016)
A. Munir, M. Sirajuddin, M. Zubair, A. Haider, S.A. Tirmizi, S. Ali, H. Khan, K. Ullah, I. Aziz, Russ. J. Gen. Chem. 87, 2380 (2017)
A. Rehman, M.I. Choudhary, W.J. Thomsen, Bioassay Techniques for Drug Development (Harwood Academic Publishers, Amsterdam, 2001), p. 9
S.T. Hafeez, M.N. Tahir, S. Ali, M. Iqbal, H. Gulab, K.S. Munawar, J. Coord. Chem. 68, 3636 (2015)
L. Findoráková, K. Győryová, M. Melník, M. Koman, F.A. Nour El-Dien. J. Coord. Chem. 63, 3348 (2010)
D. Dey, S. Roy, R.N.D. Purkayastha, R. Pallepogu, L. Male, V. Mckee, J. Coord. Chem. 64, 1165 (2011)
V. Zelěn’ak, Z. Vargov´, K. Györyov´, Spectrochim. Acta Part A 66, 262 (2007)
G.B. Deacon, R.J. Phillips, Coord. Chem. Rev. 33, 227 (1980)
M. Sirajuddin, V. Mckee, M. Tariq, S. Ali, Eur. J. Med. Chem. 143, 1903 (2018)
M. Sirajuddin, S. Ali, V. McKee, S. Zaib, J. Iqbal, RSC Adv. 4, 57505 (2014)
T.Q. Liu, R. Guo, Chin. J. Chem. 24, 620 (2006)
M. Zubair, M. Sirajuddin, A. Haider, K. Ullah, I. Ullah, Inorg. Chim. Acta 482, 567 (2018)
F.A. Shah, A.M. Khan, S. Sabir, S. Ali, Colloid Polym. Sci. 294, 87 (2015)
M. Sirajuddin, S. Ali, S. Zaib, J. Iqbal, M.N. Tahir, T.B. Hadda, Inorg. Chem. Acta 427, 178 (2015)
H.A. Benesi, J.H. Hildebrand, J. Am. Chem. Soc. 71, 2703 (1949)
G.Y. Bai, B. Dong, Y.Y. Lü, K.Z. Wang, L.P. Jin, L.H. Gao, J. Inorg. Biochem. 98, 2011 (2004)
M. Jiang, Y.T. Li, Z.Y. Wu, J. Coord. Chem. 65, 1858 (2012)
S.T. Hafeez, S. Ali, M.N. Tahir, M. Iqbal, K.S. Munawar, J. Coord. Chem. 67, 2479 (2014)
M. Nandy, D.L. Hughes, G.M. Rosair, R.K.B. Singh, S. Mitra, J. Coord. Chem. 67, 3335 (2014)
M. Tariq, N. Muhammad, M. Sirajuddin, S. Ali, N.A. Shah, M.R. Khan, M.N. Tahir, J. Organomet. Chem. 723, 79 (2013)
F.A. Shah, M. Sirajuddin, S. Ali, S.M. Abbas, M.N. Tahir, C. Rizzoli, Inorg. Chim. Acta 400, 159 (2013)
S. Roy, S. Saha, R. Majumdar, R.R. Dighe, A.R. Chakravarty, Polyhedron 29, 2787 (2010)
C.W. Jiang, H. Chao, X.L. Hong, H. Li, W.J. Mei, L.N. Ji, Inorg. Chem. Commun. 6, 773 (2003)
W.W. Davis, T.R. Stout. Appl. Microbiol. 22, 659 (1971)
A. Ambarwati, Biodiversitas 8, 320 (2007)
R.K. Alavijeh, S. Beheshti, K. Akhbari, A. Morsali, Polyhedron 156, 257 (2018)
M. Sirajuddin, S. Ali, A. Haider, N.A. Shah, A. Shah, M.R. Khan, Polyhedron 40, 19 (2012)
Acknowledgements
Higher Education Commission Islamabad Pakistan is highly acknowledgment by M. Sirajuddin for financial aid, Grant No. 6796/KPK/NRPU/R&D/HEC/2016.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ullah, K., Sirajuddin, M., Zubair, M. et al. Designing of homo and heteroleptic zinc(II) carboxylates: synthesis, spectroscopic characterizations, DNA binding study, CTAB interaction and in vitro antimicrobial evaluations. J IRAN CHEM SOC 16, 1163–1177 (2019). https://doi.org/10.1007/s13738-019-01594-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01594-6