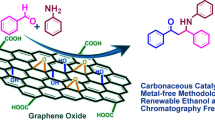
Abstract
Graphene oxide (GO), an inexpensive, environment-friendly, and metal-free carbocatalyst, used for the N-formylation of amines is developed. In this reaction, GO shows good activity, selectivity, and recyclability. This strategy has an array of advantages, such as being metal free, without additive, wide-scope protocol, scalable with a low catalyst loading of 3 wt%, use of readily available and recyclable carbocatalyst, and DMF as a readily available formyl source. Furthermore, this strategy provides an avenue for the convenient hydroformylation of various amines.
Graphical abstract






Similar content being viewed by others
References
G. Centi, S. Perathoner, D.S. Su, Catal. Surv. Asia 18, 149 (2014)
D. Chen, A. Holmen, Z. Sui, X. Zhou, Chin. J. Catal. 35, 824 (2014)
D.R. Dreyer, A.D. Todd, C.W. Bielawski, Chem. Soc. Rev. 43, 5288 (2014)
D.S. Su, S. Perathoner, G. Centi, Chem. Rev. 113, 5782 (2013)
D.R. Dreyer, C.W. Bielawski, Chem. Sci. 2, 1233 (2011)
D.S. Su, J. Zhang, B. Frank, A. Thomas, X. Wang, J. Paraknowitsch, R. Schlögl, ChemSusChem 3, 169 (2010)
P. Serp, M. Corrias, P. Kalck, Appl. Catal. A 253, 337 (2003)
A. Schaetz, M. Zeltner, W.J. Stark, ACS Catal. 2, 1267 (2012)
D.S. Su, G. Wen, S. Wu, F. Peng, R. Schlögl, Angew. Chem. 129, 956 (2017)
D.S. Su, G. Wen, S. Wu, F. Peng, R. Schlögl, Angew. Chem. Int. Ed. 56, 936 (2017)
P. Tang, G. Hu, M. Li, D. Ma, ACS Catal. 6, 6948 (2016)
C. Su, K.P. Loh, Acc. Chem. Res. 46, 2275 (2013)
Y. Gao, D. Ma, C. Wang, J. Guan, X. Bao, Chem. Commun. 47, 2432 (2011)
D.R. Dreyer, H.-P. Jia, C.W. Bielawski, Angew. Chem. Int. Ed. 122, 6965 (2010)
D.R. Dreyer, H.-P. Jia, C.W. Bielawski, Angew. Chem. Int. Ed. 49, 6813 (2010)
H.-P. Jia, D.R. Dreyer, C.W. Bielawski, Tetrahedron 67, 4431 (2011)
D.R. Dreyer, H.-P. Jia, A.D. Todd, J. Geng, C.W. Bielawski, Org. Biomol. Chem. 9, 7292 (2011)
G. Lv, H. Wang, Y. Yang, T. Deng, C. Chen, Y. Zhu, X. Hou, ACS Catal. 5, 5636 (2015)
Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D.S. Su, J. Wang, X. Bao, D. Ma, Angew. Chem. 125, 2163 (2013)
Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D.S. Su, J. Wang, X. Bao, D. Ma, Angew. Chem. Int. Ed. 52, 2109 (2013)
J.-H. Yang, G. Sun, Y. Gao, H. Zhao, P. Tang, J. Tan, A.-H. Lu, D. Ma, Energy Environ. Sci. 6, 793 (2013)
H. Huang, J. Huang, Y.-M. Liu, H.-Y. He, Y. Cao, K.-N. Fan, Green Chem. 14, 930 (2012)
C. Su, M. Acik, K. Takai, J. Lu, S. Hao, Y. Zheng, P. Wu, Q. Bao, T. Enoki, Y.J. Chabal, K.P. Loh, Nat. Commun. 3, 1298 (2012)
F. Hu, M. Patel, F. Luo, C. Flach, R. Mendelsohn, E. Garfunkel, H. He, M. Szostak, J. Am. Chem. Soc. 137, 14473 (2015)
A.V. Kumar, K.R. Rao, Tetrahedron Lett. 52, 5188 (2011)
Y. Gao, P. Tang, H. Zhou, W. Zhang, H. Yang, N. Yan, G. Hu, D. Mei, J. Wang, D. Ma, Angew. Chem. 128, 3176 (2016)
Y. Gao, P. Tang, H. Zhou, W. Zhang, H. Yang, N. Yan, G. Hu, D. Mei, J. Wang, D. Ma, Angew. Chem. Int. Ed. 55, 3124 (2016)
A. Dhakshinamoorthy, A. Primo, P. Concepcion, M. Alvaro, H. Garcia, Chem. Eur. J. 19, 7547 (2013)
H.-P. Jia, D.R. Dreyer, C.W. Bielawski, Adv. Synth. Catal. 353, 528 (2011)
R. Hett, Q.K. Fang, Y. Gao, S.A. Wald, C.H. Senanayake, Org. Process Res. Dev. 2, 96 (1998)
R.A. Forsch, A. Rosowsky, J. Org. Chem. 50, 2582 (1985)
G. Ma, M. Zancanella, Y. Oyola, R.D. Richardson, J.W. Smith, D. Romo, Org. Lett. 8, 4497 (2006)
B.-C. Chen, M.S. Bednarz, R. Zhao, J.E. Sundeen, P. Chen, Z. Shen, A.P. Skoumbourdis, J.C. Barrish, Tetrahedron Lett. 41, 5453 (2000)
P.G.M. Wuts, T. Greene, Greene’s Protective Groups in Organic Synthesis, 4th edn. (Wiley, Hoboken, 2007), p. 774
T.W. Green, P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd edn. (Wiley-Interscience, New York, 1999)
J.C. Sheehan, D.D.H. Yang, J. Am. Chem. Soc. 80, 1154 (1958)
M. Keita, M. Vandamme, O. Mahe, J.-F. Paquin, Tetrahedron Lett. 56, 461 (2015)
X. Wang, Q.-G. Wang, Q.-L. Luo, Synthesis 47, 49 (2015)
I. Ugi, Angew. Chem. 94, 826 (1982)
I. Ugi, Angew. Chem. Int. Ed. 21, 810 (1982)
U. Schollkopf, Angew. Chem. 89, 351 (1977)
U. Schollkopf, Angew. Chem. Int. Ed. 16, 339 (1977)
A. Jackson, O. Meth-Cohn, J. Chem. Soc. Chem. Commun. 1995, 1319 (1995)
F. Effenberger, J. Eichhorn, Tetrahedron Asymmetry 8, 469 (1997)
I. Ugi, U. Fetzer, U. Eholzer, H. Knupfer, K. Offermann, Angew. Chem. 77, 492 (1965)
I. Ugi, U. Fetzer, U. Eholzer, H. Knupfer, K. Offermann, Angew. Chem. Int. Ed. 4, 472 (1965)
M. Waki, J. Meienhofer, J. Org. Chem. 42, 2019 (1977)
S.B. Jagtap, S.B. Tsogoeva, Chem. Commun. 2006, 4747 (2006)
S. Kobayashi, K. Nishio, J. Org. Chem. 59, 6620 (1994)
S. Jones, C.J.A. Warner, Org. Biomol. Chem. 10, 2189 (2012)
Y. Wen, Y. Xiong, L. Chang, J. Huang, X. Liu, X. Feng, J. Org. Chem. 72, 7715 (2007)
S. Wei, K.A. Stingl, K.M. Weiß, S.B. Tsogoeva, Synlett. 2010, 707 (2010)
C.J. Gerack, L. McElwee-White, Molecules 19, 7689 (2014)
M. Lei, L. Ma, L. Hu, Tetrahedron Lett. 51, 4186 (2010)
S. Majumdar, J. De, J. Hossain, A. Basak, Tetrahedron Lett. 54, 262 (2013)
J.-G. Kim, D. Jang, Synlett 2010, 2093 (2010)
N. Ortega, C. Richter, F. Glorius, Org. Lett. 15, 1776 (2013)
S. Tanaka, T. Minato, E. Ito, M. Hara, Y. Kim, Y. Yamamoto, N. Asao, Chem. Eur. J. 19, 11832 (2013)
B. Kang, S.H. Hong, Adv. Synth. Catal. 357, 834 (2015)
C.L. Allen, B.N. Atkinson, J.M.J. Williams, Angew. Chem. 124, 1412 (2012)
C.L. Allen, B.N. Atkinson, J.M.J. Williams, Angew. Chem. Int. Ed. 51, 1383 (2012)
B.N. Atkinson, A.R. Chhatwal, H.V. Lomax, J.W. Walton, J.M.J. Williams, Chem. Commun. 48, 11626 (2012)
S. Joseph, P. Das, B. Srivastava, H. Nizar, M. Prasad, Tetrahedron Lett. 54, 929 (2013)
A.E. Pasqua, M. Matheson, A.L. Sewell, R. Marquez, Org. Process Res. Dev. 15, 467 (2011)
C. Zhang, Z.J. Xu, T. Shen, G.L. Wu, L.R. Zhang, N. Jiao, Org. Lett. 14, 2362 (2012)
X. Li, K. Liu, X. Xu, L. Ma, H. Wang, D. Jiang, Q. Zhang, C. Lu, Chem. Commun. 47, 7860 (2011)
W. Li, X.-F. Wu, Chem. Eur. J. 21, 14943 (2015)
T.V.Q. Nguyen, W.-J. Yoo, S. Kobayashi, Angew. Chem. 127, 9341 (2015)
T.V.Q. Nguyen, W.-J. Yoo, S. Kobayashi, Angew. Chem. Int. Ed. 54, 9209 (2015)
L. Zhang, Z. Han, X. Zhao, Z. Wang, K. Ding, Angew. Chem. 127, 6284 (2015)
L. Zhang, Z. Han, X. Zhao, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 54, 6186 (2015)
X. Cui, Y. Zhang, Y. Deng, F. Shi, Chem. Commun. 50, 189 (2014)
S. Kumar, S.L. Jain, RSC Adv. 4, 64277 (2014)
T.M.E. Dine, D. Evans, J. Rouden, J. Blanchet, Chem. Eur. J. 22, 5894 (2016)
H. Sheng, R. Zeng, W. Wang, S. Luo, Y. Feng, J. Liu, W. Chen, M. Zhu, Q. Guo, Adv. Synth. Catal. 359, 302 (2017)
Y. Wang, F. Wang, C. Zhang, J. Zhang, M. Li, J. Xu, Chem. Commun. 50, 2438 (2014)
Y. Wang, J. Zhang, J. Liu, C. Zhang, Z. Zhang, J. Xu, S. Xu, F. Wang, F. Wang, ChemSusChem 8, 2066 (2015)
R.M. Lanigan, P. Starkov, T.D. Sheppard, J. Org. Chem. 78, 4512 (2013)
P.B. Thale, P.N. Borase, G.S. Shankarling, RSC Adv. 6, 52724 (2016)
L. Becerra-Figueroa, A. Ojeda-Porras, D. Gamba-Sánchez, J. Org. Chem. 79, 4544 (2014)
S.N. Rao, D.C. Mohan, S. Adimurthy, Org. Lett. 15, 1496 (2013)
T.B. Nguyen, J. Sorres, M.Q. Tran, L. Ermolenko, A. Al-Mourabit, Org. Lett. 14, 3202 (2012)
M. Suchý, A.A.H. Elmehriki, R.H.E. Hudson, Org. Lett. 13, 3952 (2011)
D.D. Wirth, S.W. Baertschi, R.A. Johnson, S.R. Maple, M.S. Miller, D.K. Hallenbeck, S.M. Gregg, J. Pharm. Sci. 87, 31 (1998)
D. Stubba, G. Lahm, M. Geffe, J.W. Runyon, A.J. Arduengo III, T. Opatz, Angew. Chem. 127, 14394 (2015)
D. Stubba, G. Lahm, M. Geffe, J.W. Runyon, A.J. Arduengo III, T. Opatz, Angew. Chem. Int. Ed. 54, 14187 (2015)
J. Zhang, S. Chen, F. Chen, W. Xu, G.-J. Deng, H. Gong, Adv. Syth. Catal. 359, 2358 (2017)
A.E. Wendlandt, S.S. Stahl, J. Am. Chem. Soc. 136, 506 (2014)
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21402168), Scientific Research Foundation of Hunan Provincial Education Department (No. 15B232), National Natural Science Foundation of Hunan Province (No. 2018JJ2389), and Hunan 2011 Collaborative Innovation Center of Chemical Engineering & Technology with Environmental Benignity and Effective Resource Utilization for their support of our research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, J., Zhang, J., Zhou, X. et al. N-formylation of amine using graphene oxide as a sole recyclable metal-free carbocatalyst. J IRAN CHEM SOC 15, 2851–2860 (2018). https://doi.org/10.1007/s13738-018-1471-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1471-3