Abstract
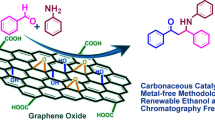
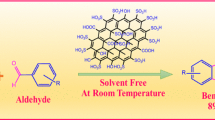
We describe an efficient, clean and metal-free procedure for the synthesis of amides via oxidative amidation of aldehydes with anilines using graphene oxide (GO) as a recyclable catalyst and KBrO3 as a mild oxidant in aqueous medium under microwave irradiation. GO nanosheets were prepared and characterized by XRD, TEM, SEM, and FT-IR, analyses. GO showed high compatibility with KBrO3 in water and offered high TOF value (1.30 × 10−3 mol g−1 min−1). GO oxygen functionalities catalyze the oxidative amidation effectively in mild condition with high recyclability. A plausible mechanism was proposed by the isolating the intermediate.
Graphic Abstract








Similar content being viewed by others
References
Greenberg A, Breneman CM (2000) The amide linkage: Structural significance in chemistry. Biochemistry and material science. Wiley-VCH, New York
Cupido T, Tulla-Puche J, Spengler J, Albericio F (2007) Curr Opin Drug Discov Dev 10:768–783
Ghose AK, Viswanadhan VN, Wendoloski JJ (1999) J Comb Chem 1:55–68
Valeur E, Bradley M (2009) Chem Soc Rev 38:606–631
Yoo WJ, Li CJ (2006) J Am Chem Soc 128:13064–13065
Seo Y, Marks TJ (2008) Org Lett 10:317–319
Ghosh SC, Ngiam JSY, Seayad AM, Tuan DT, Chai CLL, Chen A (2012) J Org Chem 77:8007–8015
Li Y, Jia F, Li Z (2013) Chem Eur J 19:82–86
Whittaker AM, Dong VM (2015) Angew Chem Int Ed 54:1312–1315
Bhattacharya S, Ghosh P, Basu B (2018) Tetrahedron Lett 59:899–903
Acosta-Guzmán P, Mateus-Gómez A, Gamba-Sánchez D (2018) Molecules 23:2309–2382
Mahesh M, Panduranga V, Prabhu G, Kumar R, Venkata Ramana P, Sureshbabu VV (2017) Synth Commun 47:716–721
Lee J, Jaeyun K, Taeghwan H (2006) Adv Mater 18:2073–2094
Rodriguez NM, Chambers A, Terry R, Baker K (1995) Langmuir 11:3862–3866
Zhu Y, Murali S, Cai W, Li X, Suk JW (2010) Adv Mater 22:3906–3924
Loh KP, Qiaoliang B, Goki E, Manish C (2010) Nat Chem 2:1015–1024
Compton OC, SonBinh TN (2010) Small 6:711–723
Jia HP, Dreyer DR, Bielawski CW (2011) Tetrahedron 67:4431–4434
Dreyer DR, Jia HP, Bielawski CW (2010) Angew Chem Int Ed 49:6813–6816
Dreyer DR, Jia HP, Todd AD, Geng J, Bielawski CW (2011) Org Biomol Chem 9:7292–7295
Jia HP, Dreyer DR, Bielawski CW (2011) Adv Synth Catal 353:528–532
Sengupta D, Ghosh S, Basu B (2017) Curr Org Chem 21:834–854
Olivier SM, Li CJ (2012) Chem Soc Rev 41:1415–1427
Brahmayya M, Suen S-Y, Dai SA (2018) J Taiwan Inst Chem Eng 83:174–183
Kumari S, Shekhar A, Mungse HP, Khatri OP, Pathak DD (2014) RSC Adv 4:41690–41695
Mirza-Aghayan M, Ganjbakhsh N, Tavana MM, Boukherroub R (2016) Ultrason Sonochem 32:37–43
Rostamnia S, Doustkhah E, Golchin-Hosseini H, Zeynizadeh B, Xin H, Luque R (2016) Catal. Sci Technol 6:4124–4133
Soul J-F, Miyamura H, Kobayashi S (2013) Chem Asian J 8:2614–2626
Dandia A, Sharma A, Parewa V, Kumawat B, Rathore KS, Sharma A (1902) RSC Adv 5(2015):91888–91889
Dandia A, Parewa V, Kumari S, Bansal S, Sharma A (2016) Green Chem 18:2488–2499
Dandia A, Gupta SL, Indora A, Saini P, Parewa V, Rathore KS (2017) Tetrahedron Lett 58:1170–1175
Dandia A, Parewa V, Rathore KS (2012) Catal Commun 28:90–94
Dandia A, Parewa V, Gupta SL, Sharma A, Rathore KS, Sharma A, Jain A (2015) Catal Commun 61:88–91
Qian Z, Cheng Y, Zhou X, Wu J, Xu G (2013) J Colloid Interface Sci 397:103–107
Paredes JI, Villar-Rodil S, SolisFernandez P, Martinez-Alonso A, Tascon JMD (2009) Langmuir 25:5957–5968
Wei D, Hoseney RC (1995) Cereal Chem 72:58–63
Polshettiwar V, Varma RS (2010) Green Chem 12:743–754
Chemat F, Esveld DC, Poux M, Di-Martino JL (1998) J Microwa Power Electromagn Energy 33:88–94
Lattes A, Oliveros E, Riviere M, Belzecki C, Mostowicz D, Abramskj W, Piccinni-Leopardi C, Germain GV, Meerssche M (1982) J Am Chem Soc 104:3929–3934
Wenglowsky S, Hegedus LSJ (1998) J Am Chem Soc 120:12468–12473
Aube J (1997) Chem Soc Rev 26:269–277
Leung CH, Voutchkova AM, Crabtree RH, Balcells D, Eisenstein O (2007) Green Chem 9:976–979
Acknowledgements
Financial assistance from the DST-SERB, CSIR, and UGC New Delhi is gratefully acknowledged. We are thankful to the Malaviya National Institute of Technology, Jaipur for the spectral analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dandia, A., Parihar, S., Saini, P. et al. Metal-Free Sustainable Synthesis of Amides via Oxidative Amidation Using Graphene Oxide as Carbocatalyst in Aqueous Medium. Catal Lett 149, 3169–3175 (2019). https://doi.org/10.1007/s10562-019-02878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02878-5