Abstract
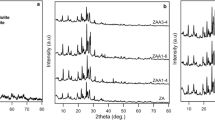
Several identification techniques have been used to study the effect of the preparation method of dealuminated Y zeolite on catalyst loading. In this paper, NaY zeolite was dealuminated by chemical operation with ethylenediaminetetraacetic acid (H4EDTA) treatment. In this method, extra-framework aluminum species removed from the supercage of zeolite and therefore increases the Si/Al ratio and pore volume. Consequently, the loading of the molybdophosphoric acid (MPA) in the supercage of zeolite is increases (0.1 g/g equal 0.049 mmol/g support) in EDTA treatment (MPA-MDAZY) in comparison with (0.0875 g/g equal 0.043 mmol/g support) in hydrothermal method (MPA-DAZY). These results were also confirmed by XRF, AAS, FTIR, SEM, BET, XRD and WDX analysis. Reducing of the reaction time and increasing of the catalytic activity of EDTA treatment toward hydrothermal method can be related to the high catalyst loading based on removing of extra-framework aluminum. The catalytic activity of two catalysts has been compared in the xanthenes synthesis reactions.








Similar content being viewed by others
References
N. Mizuno, M. Misono, Chem. Rev. 98, 199–217 (1998)
L. Marosi, C.O. Areán, J. Catal. 213, 235–240 (2003)
J. Wang, Z. Lin, S.Y. Han, M. Eum, C.W. Lee, J. Ind. Eng. Chem. 9, 281–286 (2003)
A. Thomas, C. Dablemont, J. Basset, F. Lefebvre, C. R. Chimie. 8, 1969–1974 (2005)
L. Shen, Y. Feng, H. Yin, A. Wang, L. Yu, T. Jiang, Y. Shen, Z. Wu, J. Ind. Eng. Chem. 17, 484–492 (2011)
E. O-Islas, T. López, R. Gómez, J. Navarrete, D.H. Aguilar, P. Quintana, M. Picquart, Appl. Surf. Sci. 252, 839–846 (2005)
A.V. Ivanov, T.V. Vasina, V.D. Nissenbaum, L.M. Kustov, M.N. Timofeeva, J.I. Houzvicka, Appl. Catal. A: Gen. 259, 65–72 (2004)
S. Anandan, S.Y. Ryu, W. Cho, M. Yoon, J. Mol. Catal. A: Chem. 195, 201–208 (2003)
B.R. Jermy, A. Pandurangan, Appl. Catal. A: Gen. 295, 185–192 (2005)
J. Haber, K. Pamin, J. Połtowicz, J. Mol. Catal. A: Chem. 224, 153–159 (2004)
B. Sulikowski, R. Rachwalik, Appl. Catal. A: Gen. 256, 173–182 (2003)
M. Ahmad, S.M.J. Zaidi, S.U. Rahman, S. Ahmad, Micropor. Mesopor. Mater. 91, 296–304 (2006)
A. Angelis, S. Amarilli, D. Berti, L. Montanari, C. Perego, J. Mol. Catal. A: Chem. 146, 37–44 (1999)
H. Kim, J.C. Jung, S.H. Yeom, K.Y. Lee, I.K. Song, J. Mol. Catal. A: Chem. 248, 21–25 (2006)
P. He, B. Xu, X. Xu, L. Song, X. Wang, Chem. Sci. 7, 1011–1015 (2016)
J.W. Zhao, Y.Z. Li, L.J. Chen, G.Y. Yang, Chem. Commun. 52, 4418–4445 (2016)
X. Xu, S. Chen, Y. Chen, H. Sun, L. Song, W. He, X. Wang, Small (2016). doi:10.1002/Small.201503695
S. Rayati, F. Salehi, J. Iran. Chem. Soc. 12, 309–315 (2015)
M. Moosavifar, A.N. Arbat, Z. Rezvani, K. Nejati, Chin. J. Catal 36, 1719–1725 (2015)
S.R. Mukai, T. Masuno, I. Ogina, K. Hashimoto, Appl. Catal. A: Gen. 165, 219–226 (1997)
J. Wang, D. Jiang, J.O. Baeg, C.W. Lee, J. Ind. Eng. Chem. 10, 454–459 (2004)
H.V. Bekkum, E.M. Flanigen, P.A. Jacobs, J.C. Jansen (eds.), Introduction to Zeolite Science and Practice, 2nd edn. (Elsevier Science, 2001), p. 150
M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moosavifar, Appl. Catal. A Gen. 358, 157–163 (2009)
F. Shirini, M. Abedini, S. Akbari-Dadamahaleh, A. Rahmaninia, J. Iran. Chem. Soc. 11, 791–824 (2014)
M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moosavifar, C. R. Chimie. 14, 489–495 (2011)
M. Moosavifar, S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, C. R. Chimie. 14, 953–956 (2011)
B. Sulikowski, J. Phys. Chem. 97, 1420–1425 (1993)
A. Zhang, C. Li, S. Bao, Q. Xu, Micropor. Mesopor. Mat. 29, 383–388 (1999)
V. Solinas, I. Ferino, Catal. Today 41, 179–189 (1998)
Z. Mehraban, F. Farzaneh, Micropor. Mesopor. Mat. 88, 84–90 (2006)
A. Derkowski, W. Franus, H.W. Nowicka, A. Czímerová, Int. J. Miner. Process. 82, 57–68 (2007)
G. Øye, J. Sjöblom, M. Stöcker, Adv Colloid Interf. Sci. 89–90, 439–466 (2001)
Q.-H. Xia, K. Hidajat, S. Kawi, Mater. Lett. 42, 102–107 (2000)
M. Moosavifar, S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor Baltork, J. Mol. Catal. A: Chem. 377, 92–101 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moosavifar, M., Fathyunes, L. Influence of the post-synthesis method on the number and size of secondary mesoporous structure of NaY zeolite and its effect on catalyst loading. An efficient and eco-friendly catalyst for synthesis of xanthenes under conventional heating. J IRAN CHEM SOC 13, 2113–2120 (2016). https://doi.org/10.1007/s13738-016-0929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0929-4