Abstract
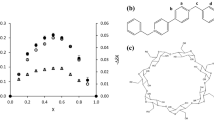
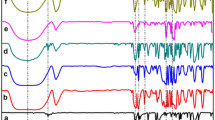
Formation of the complex of ethyl viologen in its cationic (Ev+•) and neutral (Ev∘) forms with β-cyclodextrin (β-CD) was investigated by means of voltammetric technique in buffer solution of pH 7.00. The number of βCD (n or m) per viologen species (Ev+•) or (Ev∘), bonding equilibrium constants as well as bonding rate constants was calculated. The calculated values of \(K_{\text{eq}}^{(1)}\) and \(K_{\text{eq}}^{ ( 2)}\) (pertaining to the bonding of Ev+• and Ev∘ with βCD) are 13.6 M–n and 2.1 × 103 M−m, respectively, whereas the calculated values of n and m are 0.54 and 1.25, respectively. The bimolecular rate constant for the Ev∘−βCD inclusion complex formation is 3.03 × 103 M−1s−1. These results are supported by the simulation of the experimental cyclic voltammograms. This study also highlights the significance of the proposed electrochemical method as compared to earlier studies on viologen-Cyclodextrin systems.









Similar content being viewed by others
References
W. Saenger, Angew. Chem. Int. Ed. Engl. 19, 344 (1980)
C. Bird, A.T. Kuhn, Chem. Soc. Rev. 10, 49 (1981)
P.M. Bersier, J. Bersier, B. Klingert, Electroanalysis 3, 443 (1991)
A. Yasuda, H. Mori, J. Seto, J. Appl. Electrochem. 17, 567 (1987)
A. Yasuda, J. Seto, J. Appl. Electrochem. 18, 333 (1988)
A. Mirzoian, A.E. Kaifer, Chem. Eur. J. 3, 1052 (1997)
A. Mirzoian, Solvent effects and redox control on host-guest binding phenomena. Ph.D. Dissertation, University of Miami, 1998
T. Matsue, T. Kato, U. Akiba, T. Osa, Chem. Lett. 1825 (1985)
M. Tariq, Electrochemical production of bromine atom free radical and its reactions with biologically active and important compounds. Ph.D. thesis, Karachi University, 2010
M. Mohammad, M. Tariq, M.T. Somroo, Collect. Czech Chem. Commun. 75, 1061 (2010)
R.S. Nicholson, I. Shain, Anal. Chem. 36, 706 (1964)
A.J. Bard, L.R. Faulkner, Electrochemical methods—fundamentals and applications, (Wiley, New York, 2001)
C. Lee, B.Y. Kim, J.W. Park, Anal. Sci. 17, a69 (2001)
J.Y. Kim, C. Lee, J.W. Park, J. Electroanal. Chem. 504, 104 (2001)
Z. Galus, Fundamentals of Electrochemical Analysis, Chap 14 (Ellis Horwood, Chichester, 1976)
N. Gupta, H.L. Linshitz, J. Am. Chem. Soc. 119, 6384 (1997)
M. Mohammad, L. Naz, A. Rauf, S. Rauf, J. Chem. Soc. Pak. 34, 659 (2013)
Acknowledgments
Shaz Akber is thankful to Dr. M Tariq and Dr. Lubna Naz, ICCBS Karachi University for their useful suggestions and help in connection with the digital simulation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbar, S., Naqvi, I.I. & Mohammad, M. Electron transfer reactions associated with ethyl viologen-β-cyclodextrin complexation: equilibrium and kinetic aspects. J IRAN CHEM SOC 11, 615–621 (2014). https://doi.org/10.1007/s13738-013-0331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0331-4