Abstract
A 77-year-old male patient on maintenance hemodialysis therapy who underwent unilateral adrenonephrectomy 9 years ago was transferred to our hospital after 4 months of acute treatment for skull base osteomyelitis. He presented with unexplained hypotension during dialysis sessions. Further evaluation led to a diagnosis of primary adrenal insufficiency, followed by the start of oral hydrocortisone. Six months after admission, the patient was found to have a positive COVID-19 result on a rapid antigen test and mild symptoms. The patient complained of fatigue after the disappearance of the symptoms. Subsequently, the systolic blood pressure gradually declined despite the additional administration of fludrocortisone and caused difficulties in undergoing hemodialysis. The patient’s lasting fatigue raised a suspicion of post-COVID-19 syndrome, requiring larger dosages of corticosteroids by stress dosing. Hypotension was interpreted as a symptom associated with adrenal insufficiency. The dosages of corticosteroids were increased beyond the upper recommended limits. The effect eventually stabilized the patient’s hemodynamics. Hydrocortisone was increased as follows: 35 mg/day for nondialysis days and 55 mg/day for dialysis days, divided into three or four doses per day (20 mg in the morning, 20 mg before dialysis, 10 mg in the afternoon, and 5 mg in the evening). The dosage of fludrocortisone was increased up to 0.5 mg/day. In conclusion, the requirement for corticosteroids significantly increased in association with post-COVID-19 syndrome. The management of patients with adrenal insufficiency in the context of concomitant post-COVID-19 syndrome needs further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has spread worldwide and raised concerns about the postacute effects of the infection in addition to its acute manifestations [1]. Various physical and neuropsychiatric symptoms may appear during or after the infection and last for months or longer, which is known as post-COVID-19 syndrome or long COVID. The most common symptoms include fatigue, shortness of breath, arthralgia, chest pain, sleep difficulties, and cognitive impairment. Persistent symptoms significantly reduce health-related quality of life and frequently require further medical intervention.
The management of primary adrenal insufficiency remains a challenge, as no objective assessment has been proven to be reliable for monitoring corticosteroid replacement therapy [2]. The recommended corticosteroid replacement therapy consists of multiple daily doses of hydrocortisone (15–25 mg or 7.5–15 mg/m2/day) or cortisone acetate (20–35 mg or 20 mg/m2/day), corresponding to a mean cortisol production rate of 5–11 mg/m2/day, combined with fludrocortisone (0.05–0.2 mg) [2, 3]. For the prevention of adrenal crisis, supplemental doses of hydrocortisone are required according to specific stress-associated situations. Targeting adrenocorticotropic hormone (ACTH) or plasma renin activity (PRA) values within the normal range is known to lead to overreplacement. Thus, physicians must modulate corticosteroid doses based primarily on clinical assessment while considering over- or under-replacement. Cardiovascular, malignant, and infectious diseases are the leading causes of death in patients with adrenal insufficiency, while premature death from adrenal crises is still a frequent problem [3, 4].
The present report describes a patient with end-stage renal disease requiring hemodialysis and primary adrenal insufficiency, in whom lasting fatigue following acute COVID-19 illness led to a significant increase in the requirement of corticosteroids.
Case report
A 77-year-old man with a 32-year history of hemodialysis was transferred to our long-term care hospital from an acute care hospital after 4 months of acute treatment for skull base osteomyelitis accompanied by dysphagia requiring nasogastric feeding. Preexisting hypotension during hemodialysis sessions worsened within the last month, along with insufficient fluid removal despite bilateral pleural effusion, with a larger amount on the right side. His medical history was notable for aortic valve replacement for severe aortic stenosis 7 months ago, catheter ablation for atrial fibrillation 4 years ago, left radical nephrectomy for kidney cancer 9 years ago, and hypothyroidism requiring thyroid hormone replacement 15 years ago. The cause of renal insufficiency was unknown. His blood pressure was 152/81 mmHg, his pulse was 76 beats/min and regular, and his body temperature was 36.3 °C.
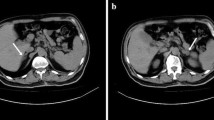
At the first dialysis session in our hospital, the systolic blood pressure decreased to approximately 70 mmHg despite the use of etilefrine hydrochloride, a vasopressor drug that is generally used in Japanese dialysis units, resulting in discontinuation of the dialysis session in less than 3 h, with approximately one liter of fluid remaining. The cessation led to a rise in blood pressure to the predialysis level. Echocardiography showed normal left ventricular systolic function. Laboratory tests revealed mild hyponatremia (Na: 133 mmEq/L), eosinophilia (absolute eosinophils: 877/μL), and hypercalcemia (corrected Ca: 11.3 mg/dL). The patient was extremely sensitive to cold. These findings, in association with the unexplained hypotension, raised a suspicion of adrenal insufficiency. At the second dialysis session, hypotension similarly appeared again under the additional use of amezinium metilsulfate. The intravenous administration of 10 mg of prednisolone, the only intravenous corticosteroid available in our hospital, promptly increased the systolic blood pressure to 140 mmHg and achieved hemodynamic stability during the rest of the dialysis session. Laboratory examinations of blood samples collected before the administration of prednisolone revealed a slightly increased adrenocorticotropic hormone (ACTH) level (83.0 pg/mL) (normal range 7.2–63.3 pg/mL). A corticotropin stimulation test failed to increase serum cortisol (baseline: 3.6 μg/dL; after 1 h: 6.0 μg/dL). A computed tomography scan showed the absence of the left kidney and adrenal gland without any evidence of other underlying comorbidities. The result of a T-SPOT. TB test was negative. The aforementioned findings and the clinical course were compatible with primary adrenal insufficiency after unilateral adrenalectomy as part of a left radical nephrectomy. The measurement of 21-hydroxylase autoantibodies was not performed, as it was not covered by the Japanese health insurance system. Oral hydrocortisone was started (20 mg/day for nondialysis days and 25 mg/day for dialysis days, corresponding to 14.2 mg/m2/day and 17.8 mg/m2/day) and divided into 2 doses per day (15 mg in the morning on nondialysis days, 20 mg in the morning on dialysis days, and 5 mg in the evening), which successfully maintained blood pressure along with the disappearance of eosinophilia and hypercalcemia. Mild hyponatremia lasted. The dry weight was adjusted to achieve a human atrial natriuretic peptide level of approximately 80 pg/mL and increased by 2.5 kg over 6 months. Chest X-ray showed gradual reduction of pleural effusion.
Six months after admission, the patient was found to have a positive COVID-19 test result on a rapid antigen test with a nasopharyngeal sample following the spread among patients hospitalized in the same room. He was fully vaccinated against COVID-19. His symptoms included a high temperature and mild cough without unusual fatigue or changes in his hemodynamic parameters. The administration of molnupiravir was followed by the decline in fever within a few days and gradual disappearance of cough. Two weeks later, assistive bathing once or twice a week was restarted. Over the next 2 weeks, the patient complained of fatigue with frequent refusal of assistive bathing, and the systolic blood pressure gradually decreased to less than 100 mmHg on nondialysis days and to approximately 80 mmHg during and after dialysis sessions. Electrocardiogram showed new ST segment depressions in the anterolateral leads, while echocardiography showed no significant abnormalities. Coronary angiography was performed in the acute care hospital and showed non-obstructive coronary arteries. After confirming hypoaldosteronism (less than the detection limit) by the measurement of serum aldosterone, fludrocortisone (0.2 mg/day) was started. The systolic blood pressure temporarily increased, however, over the next 2 months, it gradually declined again to 60 mmHg along with difficulties in undergoing hemodialysis. Electrocardiogram and echocardiography showed no new abnormalities. The results of blood culture tests were negative. Laboratory tests showed preexisting mild hyponatremia alone, without eosinophilia, hypercalcemia, liver dysfunction, or elevated C-reactive protein. A computed tomography scan of the right adrenal gland was unremarkable. No other comorbidities were identified. The dosages of hydrocortisone and fludrocortisone had already reached the upper recommended limits. Lasting fatigue after acute COVID-19 infection raised a suspicion of post-COVID-19 syndrome, requiring larger dosages of corticosteroids by stress dosing. Hypotension was interpreted as a symptom associated with adrenal insufficiency. Increasing dosages of corticosteroids were administered. When hypotension was remarkable after bathing or during the latter half of a dialysis session, hydrocortisone was increased. Hypotension before the dialysis session was followed by an increment of fludrocortisone, as the intravenous administration of 10 mg of prednisolone was ineffective in such a situation. The effect eventually increased the systolic blood pressure to between 110 and 130 mmHg and stabilized the patient’s hemodynamics. Refusal of assistive bathing faded despite lasting fatigue. Hydrocortisone was increased as follows: 35 mg/day for nondialysis days and 55 mg/day for dialysis days, divided into 3 or 4 doses per day (20 mg in the morning, 20 mg before dialysis, 10 mg in the afternoon, and 5 mg in the evening). The dosage of fludrocortisone was increased up to 0.5 mg/day. While the effect of the updosing of hydrocortisone became evident by the following day, increasing fludrocortisone required several days to demonstrate its efficacy. The patient had an uneventful course in the following 3 months with hemodynamic stability despite persistent fatigue. Figure 1 shows the clinical course during 12 months after admission.
Discussion
Adrenal function remains preserved in most patients after acute COVID-19 infection, whereas there are some case reports of adrenal insufficiency after COVID-19 infection in association with adrenal hemorrhage and infarction, dexamethasone use in the acute phase, and critical illness-related corticosteroid insufficiency [5]. Clinicians have given particular attention to the potential impact of COVID-19 infection on the adrenal gland due to several findings as follows: The ACE2 receptor and transmembrane serine protease 2, which are required for cellular access by SARS-CoV-2, are present in the adrenal cortex [6]. Autopsy studies have shown frequent adrenal lesions in patients who died of COVID-19 [7, 8]. Symptoms of long COVID resemble those of adrenal insufficiency. However, to date, there remains little evidence suggesting the impairment of corticosteroid production by SARS-CoV-2 [5]. High cortisol levels within 48 h of admission are associated with increased mortality [9]. Possible contributors to adrenal lesions in some cases include hypercoagulability and endothelial dysfunction, especially in severe COVID-19 infection, and underlying comorbidities such as antiphospholipid syndrome [5, 10].
For the prevention of adrenal crisis, patients with primary adrenal insufficiency require an increased glucocorticoid dosage according to the magnitude of stressors [2]. Doubled or tripled hydrocortisone doses are generally recommended when a patient experiences febrile illness, an infection requiring antibiotics, or a small medical procedure. The duration is usually short. In the present case, the adjusted glucocorticoid dosages, compared to the previous dosages, were 1.5 times higher for nondialysis days and more than doubled for dialysis days. The duration of increased dosing is unclear but may be over a year. A significant increase in the glucocorticoid requirement and the long administration duration may suggest the severity of exhaustion after COVID-19 infection.
Considering the larger requirement of fludrocortisone despite increased hydrocortisone dosages, the possibility of decreased mineralocorticoid sensitivity after acute COVID-19 illness might have to be investigated. When increased hydrocortisone doses are used, the mineralocorticoid requirement usually decreases due to the relatively high mineralocorticoid potency of hydrocortisone [2]. Higher fludrocortisone doses (up to 0.5 mg daily) are required in newborns and children due to their lower mineralocorticoid sensitivity compared to that of adults, as well as in the last trimester of pregnancy with high levels of progesterone exerting antimineralocorticoid actions [2, 11]. There are no generally accepted methods for the assessment of mineralocorticoid sensitivity available in the clinical setting. The precise causal factor of the increased requirement of fludrocortisone in the present case is unknown.
Aldosterone, the most important mineralocorticoid hormone, increases blood pressure by the direct activation of the sympathetic nervous system and vascular smooth muscle contraction, whereas sodium reabsorption leading to hypertension via the epithelial sodium channel of renal epithelial cells of the distal nephron is recognized as the traditional effect of aldosterone [12]. Presumably, the former effect mainly functioned in the present case, as the latter effect hardly works in anuric subjects. Aldosterone also produces endothelial and vascular smooth muscle cell dysfunction, myocardial fibrosis, and proarrhythmogenic effects. Considering such effects by aldosterone, the replacement of mineralocorticoids with fludrocortisone should be kept to the minimum.
Chronic adrenal insufficiency is a possible complication of unilateral adrenalectomy, although it is generally considered that unilateral adrenalectomy does not induce adrenal insufficiency due to compensation by the contralateral adrenal gland [13, 14]. Previous studies reported that unilateral adrenalectomy can cause impairment of the adrenocortical reserve and result in primary adrenal insufficiency associated with severe stressors [13, 15]. The present case underwent unilateral adrenonephrectomy 9 years previously, experienced severe infection requiring intensive care and subsequently developed primary adrenal insufficiency. The clinical course was compatible with a previous report.
In conclusion, the requirement for corticosteroids was significantly increased in a patient with preexisting adrenal insufficiency in association with post-COVID-19 syndrome. It was difficult to determine whether to increase the dosages of corticosteroids over the usually recommended ranges in the absence of previous similar case reports. Although dose tapering as early as possible is preferable, a long duration might be needed. The management of patients with adrenal insufficiency in the context of concomitant post-COVID-19 syndrome needs further investigation.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- COVID-19:
-
Coronavirus disease 2019
- PRA:
-
Plasma renin activity
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737–54. https://doi.org/10.1080/23744235.2021.1924397. (Epub 2021 May 22. PMID: 34024217; PMCID: PMC8146298).
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–89. https://doi.org/10.1210/jc.2015-1710. (PMID: 26760044; PMCID: PMC4880116).
Oprea A, Bonnet NCG, Pollé O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab. 2019;2(10):2042018818821294. https://doi.org/10.1177/2042018818821294. (PMID: 30746120; PMCID: PMC6360643).
Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab. 2006;91(12):4849–53. https://doi.org/10.1210/jc.2006-0076. (Epub 2006 Sep 12 PMID: 16968806).
Clarke SA, Abbara A, Dhillo WS. Impact of COVID-19 on the endocrine system: a mini-review. Endocrinology. 2020;163(1):bqab203. https://doi.org/10.1210/endocr/bqab203. (PMID: 34543404; PMCID: PMC8500009).
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052. (Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627).
Zinserling VA, Semenova NY, Markov AG, Rybalchenko OV, Wang J, Rodionov RN, Bornstein SR. Inflammatory cell infiltration of adrenals in COVID-19. Horm Metab Res. 2020;52(9):639–41. https://doi.org/10.1055/a-1191-8094. (Epub 2020 Jul 6 PMID: 32629518).
Freire Santana M, Borba MGS, Baía-da-Silva DC, Val F, Alexandre MAA, Brito-Sousa JD, Melo GC, Queiroga MVO, Leão Farias ME, Camilo CC, Naveca FG, Xavier MS, Monteiro WM, Augusto Pivoto João G, Hajjar LA, Ordi J, Lacerda MVG, Ferreira LCL. Case report: in adrenal pathology findings severe COVID-19: an autopsy study. Am J Trop Med Hyg. 2020;103(4):1604–7. https://doi.org/10.4269/ajtmh.20-0787. (PMID: 32876012; PMCID: PMC7543860).
Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, Comninos AN, Abbara A, Dhillo WS. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–60. https://doi.org/10.1016/S2213-8587(20)30216-3. (Epub 2020 Jun 18. PMID: 32563278; PMCID: PMC7302794).
Popescu M, Terzea DC, Carsote M, Ghenea AE, Costache A, Popescu IAS, Biciuşcă V, Busuioc CJ, Ghemigian AM. COVID-19 infection: from stress-related cortisol levels to adrenal glands infarction. Rom J Morphol Embryol. 2022;63(1):39–48. https://doi.org/10.47162/RJME.63.1.03. (PMID: 36074666; PMCID: PMC9593124).
Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, Falorni A, Gan EH, Hulting AL, Kasperlik-Zaluska A, Kämpe O, Løvås K, Meyer G, Pearce SH. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275(2):104–15. https://doi.org/10.1111/joim.12162. (Epub 2013 Dec 16 PMID: 24330030).
Capelli I, Gasperoni L, Ruggeri M, Donati G, Baraldi O, Sorrenti G, Caletti MT, Aiello V, Cianciolo G, La Manna G. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol. 2020;33(1):37–48. https://doi.org/10.1007/s40620-019-00600-7. (Epub 2019 Apr 15 PMID: 30989614).
Yoshiji S, Shibue K, Fujii T, Usui T, Hirota K, Taura D, Inoue M, Sone M, Yasoda A, Inagaki N. Chronic primary adrenal insufficiency after unilateral adrenonephrectomy: a case report. Medicine (Baltimore). 2017;96(51):e9091. https://doi.org/10.1097/MD.0000000000009091. (PMID: 29390437; PMCID: PMC5758139).
Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(8):727–34. https://doi.org/10.1056/NEJMra020529. (PMID: 12594318).
Yokoyama H, Tanaka M. Incidence of adrenal involvement and assessing adrenal function in patients with renal cell carcinoma: is ipsilateral adrenalectomy indispensable during radical nephrectomy? BJU Int. 2005;95(4):526–9. https://doi.org/10.1111/j.1464-410X.2005.05332.x. (PMID: 15705073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has declared that no conflict of interest exists.
Informed consent
Informed consent was obtained from the patient included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Eguchi, E. Post-COVID-19 syndrome increased the requirement for corticosteroids in a dialysis patient with preexisting adrenal insufficiency. CEN Case Rep 12, 347–351 (2023). https://doi.org/10.1007/s13730-023-00772-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-023-00772-z