Abstract
Despite the reports on glomerulonephritis associated with COVID-19 mRNA vaccines, no study has reported about the dense deposit disease (DDD). Here, we present a case of hilar lymphadenopathy after the COVID-19 mRNA vaccination, following which the patient developed tubulointerstitial nephritis (TIN) and DDD. A 74-year-old man received his second dose of mRNA vaccine, and on the next day, he developed fever, urticaria, and dyspnea. On further examination, he had pleural effusion and right hilar lymphadenopathies, which were improved with conservative therapy. After 48 days of the second vaccination, he developed renal dysfunction and new-onset hematuria. Light microscopy findings by renal biopsy revealed apparent mesangial cell proliferation, increased mesangial matrix in the glomeruli, and diffuse inflammatory cell infiltration in the interstitium. Immunofluorescence analysis revealed 1 + positive results for IgG and IgM, negative results for IgA, and 2 + positive results for C3 with a garland pattern on the capillary walls. Electron microscopy revealed that severe cell proliferation in the capillary rumen, and continuous, thickened, and highly dark-stained spotty dense deposits in the glomerular basement membrane; and noncontinuous spotty dense deposits in the tubular basement membrane. Based on the decrease in C3 and pathological findings, TIN accompanied with DDD was diagnosed. The mRNA vaccine might have contributed to the development of lymphadenopathies, TIN, and DDD in this case. Moreover, TIN and DDD might be associated with the activated alternative pathway induced by the mRNA vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis [1], minimal change disease, antiglomerular basement membrane (anti-GBM) antibody disease [2, 3], IgA nephropathy [3, 4], and tubulointerstitial nephritis (TIN) [5], which are linked to COVID-19 vaccination, have recently been reported in a growing literature on glomerulonephritis. Complement activation-related pseudoallergy (CARPA) is now recognized as the underlying cause of hypersensitivity reactions to various drugs, such as monoclonal antibody (e.g., rituximab), liposome-encapsulated products (doxil or ambisome), and micellized anticancer drug (paclitaxel) [6]. Here, we present a case of a patient who developed hilar lymphadenopathy, TIN, and dense deposit disease (DDD) following mRNA vaccination.
Case report
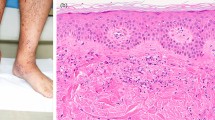
A 74-year-old man with a past medical history of hyperlipidemia received his second dose of the Pfizer-BioNTech COVID-19.vaccine (Pfizer, Inc. Philadelphia, PA) on his left upper arm on June 29, 2021. On the next day, he developed fever and urticaria on the trunk and extremities. He took 120 mg of loxoprofen sodium hydrate per day for 7 days. On July 10, 2021, he visited our hospital for gradually deteriorating dyspnea. The levels of serum creatinine (sCr) and C-reactive protein were 0.75 mg/dL and 15.0 mg/dL, respectively, and urinalysis revealed no proteinuria or red blood cells. He presented with right pleural effusion and right hilar lymphadenopathies, which indicated the presence of malignant lesions. The repeated cytological findings of pleural effusion by cellblock demonstrated a reactive pattern, and fine-needle aspiration of an enlarged lymph node revealed no evidence of malignancy; thus, lung carcinoma was denied. The level of C-reactive protein was reduced to the normal range, and lymphadenopathies gradually regressed with the administration of ampicillin/sulbactam for 10 days. After 48 days of the second vaccination, his sCr level increased to 1.31 mg/dL, and new-onset hematuria was observed. Serological tests for ANCA, anti-SSA/Ro, anti-SSB/Ro, anti-DNA, and anti-GBM antibodies were negative. There was no elevation in IgG4, angiotensin-converting enzyme, or anti-streptolysin O titer. A decrease in C3 (50.1 mg/dL; normal range, 73–138 mg/dL) along with the elevated titer of antiScl-70 antibody, anti-cardiolipin antibody, and anti-CLβ2 GP1 antibodies are reported in Table 1. Renal biopsy light microscopy findings revealed 15 glomeruli; of these, 3 were global sclerosis. Periodic acid-methenamine silver staining (PAM) revealed mild mesangial cell proliferation with focal endocapillary proliferation (Fig. 1a; arrow head) in one glomerulus. In addition, both irregularity of the glomerular basement membrane and a double contour were seen segmentally (Fig. 1a). Periodic acid-Schiff staining revealed massive and diffuse inflammatory cell infiltration in the interstitium. The majority of infiltrating cells were lymphocytes and plasma cells. However, a few eosinophilic cells were seen (Fig. 1b). No necrotizing lesions or vasculitis were observed. Immunofluorescence analysis revealed 1 + positive results for IgG (Fig. 1c) and IgM, negative results for IgA, and 2 + positive results for C3 on the capillary and mesangium area (Fig. 1d). A garland pattern was observed (magnified in the top left rectangle in Fig. 1d). Electron microscopy revealed severe glomerular cell proliferation (Fig. 2a); and continuous, thickened, and highly dark-stained spotty dense deposits in the glomerular basement membrane (Fig. 2b; blue arrows). Furthermore, the severe thickness of the basement membrane (Fig. 2c; blue arrows) with dense deposits and the mild thickness of the basement membrane (Fig. 2c; yellow arrows) with small amounts of dense deposits coexisted. The findings of noncontinuous spotty dense deposits (Fig. 2d; blue arrows) in the tubular basement membrane were also observed. The patient did not present with ocular lesions. The drug lymphocyte stimulation test for loxoprofen sodium hydrate, ampicillin/sulbactam, and atorvastatin was negative. To distinguish DDD from monoclonal immunoglobulin deposition disease, immune histochemical staining was carried out using paraffin sections. Both kappa and lambda were found to be positive in glomeruli, but there was no discernible difference between the two light chains. Finally, both serum and urine electrophoresis results indicated the absence of M protein.
Pathological findings by renal biopsy: light microscopy (a and b); immunofluorescence for IgG (c) and for C3 (d). Periodic acid-methenamine silver staining revealed focal endocapillary proliferation (a, arrow) in one glomerulus. Periodic acid-Schiff staining revealed massive and diffuse inflammatory cell infiltration in the interstitium. The majority of infiltratory cells were lymphocytes and plasma cells. A few eosinophilic cells were seen (b). Immunofluorescence analysis revealed 1 + positive results for IgG (c) and 2 + positive results for C3 on the capillary walls and mesangium area (d). A garland pattern is magnified in top left rectangle (d)
Electron microscopy revealed severe glomerular cell proliferation (a) and continuous and dark-stained spotty dense deposits (blue arrows) in the glomerular basement membrane (b); the severe thickness of the basement membrane (blue arrows) with dense deposits and the mild thickness of the basement membrane (yellow arrows) with small amounts of dense deposits coexisted (c). Noncontinuous spotty dense deposits in the tubular basement membrane (d)
Although, this case presented with several atypical findings, TIN accompanied with DDD was diagnosed based on serological and pathological findings. Prednisolone (50 mg; 0.8 mg/kg) administration was started to treat TIN. At 7 weeks after the renal biopsy, his C3 level returned to the normal range, and the patient’s renal dysfunction and urinary findings gradually improved (Table 2).
Discussion
We presented a case of a patient who developed reactive lymphadenopathy, TIN, and DDD, which was believed to be caused by strong complement amplification. Because both coincidental onset with TIN and DDD following an acute allergic response that occurred approximately before 7 weeks led us to believe that each event or disease can be associated with COVID-19 mRNA vaccination via an inflammatory or immunological response following mRNA vaccination. Although TIN caused by nonsteroidal anti-inflammatory drugs and antibiotics cannot be completely ruled out, this is a unique case as it is inconsistent with any known disease entities or clinical course.
Post-vaccination ipsilateral lymphadenopathy typically occurs in readily accessible sites, such as the cervical, axillary and supraclavicular lymph nodes [7]. In the present case, lymphadenopathy occurred on the contralateral side, which mimics lung carcinoma and compresses the intrabrachial duct causing dyspnea; this suggested the involvement of a severe immunological reaction. Although the enlarged lymph nodes degenerated after antibiotic treatment, they might be attributed to acute allergic reactions due to the mRNA vaccine rather than infections or malignancies, based on the results of several clinical tests.
DDD is associated with the deposition of complement C3 in the glomeruli and is thought to result from uncontrolled activation of an alternative signaling pathway [8]. No study has reported DDD associated with mRNA vaccination. IF revealed that IgG staining can be a slightly stronger or C3 can be a slightly weaker marker for the diagnosis of DDD in the present case. Although we could not properly explain these pathological findings, we hypothesized that the duration from the onset of this disease and/or vaccination-induced mechanisms might be related to the atypical findings.
Recently, several cases of TIN after mRNA vaccination have been reported [5]. In a mice model, Turnberg et al. demonstrated that the activation of the alternative pathway rather than classical pathway contributed to glomerular and tubulointerstitial damage [9]. Mira FS et al. [5] revealed that positive lymphocyte transformation test findings for polyethylene glycol and vaccine solution indicated T-cell involvement, which could represent a T-cell-mediated injury. Consequently, aberrant innate and acquired immune responses could be involved in the onset of interstitial nephritis [10, 11].
The SARS-CoV-2 spike protein binds to heparan sulfate on nucleated cells and enhances the complement’s alternative pathway by interfering with the binding of complement factor H, an inhibitor of the alternative pathway [12]. Adverse reactions to vaccines might develop because of the interaction between the vaccinated subject’s susceptibility and various vaccine components. The molecular resemblance between certain pathogenic elements in the vaccine and specific human proteins has been suggested as one of the mechanisms behind these reactions. This resemblance may cause immune cross reactivity, in which the immune system’s reaction to pathogenic antigens destroys similar human proteins, resulting in an autoimmune disease [10]. Although the clinical significance was unknown, the positivity for the two types of antiphospholipid antibodies and decrease in C3 suggest dysregulations of the alternative pathway of complements in this case. An alternative pathway of complement activation may explain many clinical manifestations including microangiopathy, thrombocytopenia, renal injury, and thrombophilia in patients with COVID-19. These are also observed in other complement-driven diseases such as atypical hemolytic uremic syndrome and catastrophic antiphospholipid antibody syndrome [12].
With the C3a and C5a anaphylatoxins binding to mast cells in CARPA syndrome, it has recently been recognized that several modern-day therapeutic molecules may activate complement via a non-IgE mediated mechanism, triggering the release of several vasoactive mediators that cause the clinical features associated with hypersensitivity reactions [6, 13]. Reactive lymphadenopathy, TIN, and DDD may be influenced by such mechanisms. In the present case, hematuria was observed after 7 weeks of vaccination. While the serum creatinine level gradually increased from 0.75 to 1.28 mg/dL over 7 weeks, the urine test was not performed until the day of the case report. Thus, we think that the virtual onset can be much earlier.
In conclusion, COVID-19 vaccination might contribute to the development of hilar lymphadenopathy, TIN and DDD in a patient following mRNA vaccination. Moreover, DDD and TIN might be associated with activated alternative pathway induced by COVID-19 mRNA vaccination.
References
Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:611–3.
Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100:471–2.
Tan HZ, Tan RY, Choo JCJ, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–71.
Bomback AS, Kudose S, D’Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78:477–80.
Mira FS, Costa Carvalho J, de Almeida PA, et al. A case of acute interstitial nephritis after two doses of the BNT162b2 SARS-CoV-2 vaccine. Int J Nephrol Renovasc Dis. 2021;14:421–6.
Macdougall IC, Vernon K. Complement activation-related pseudo-allergy: a fresh look at hypersensitivity reactions to intravenous iron. Am J Nephrol. 2017;45:60–2.
Cellina M, Irmici G, Carrafiello G. Unilateral axillary lymphadenopathy after coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021;216:W27.
Smith RJ, Harris CL, Pickering MC. Dense deposit disease. Mol Immunol. 2011;48:1604–10. https://doi.org/10.1016/j.molimm.2011.04.005.
Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT. Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol. 2006;177:4094–102. https://doi.org/10.4049/jimmunol.177.6.4094.
Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586–94.
Caso F, Costa L, Ruscitti P, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19: 102524.
Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–9.
Dobrovolskaia MA, McNeil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013;172:456–66. https://doi.org/10.1016/j.jconrel.2013.05.025.
Acknowledgements
We would like to thank Dr. Ehara from Shinsyu University for the diagnosis using electron microscopy. A part of this case report was presented at the 119th Japanese Society of Internal Medicine, which was held in Kyoto, Japan, on April 14, 2022.
Funding
There was no funding source for the work.
Author information
Authors and Affiliations
Contributions
Idea and design of the study, Hironori Nakamura; data acquisition, Hironori Nakamura; pathological analysis or interpretation, Hironori Nakamura and Mutsuki Makino; supervision or mentorship, Michiko Ueda, Mariko Anayama, and Yasushi Makino. Hironori Nakamura takes the responsibility that this study has been reported honestly, accurately, and transparently, as well as accepts accountability for the overall work.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no relevant financial interests.
Ethical approval
Written informed consent was obtained from the patient for the publication of the details of his medical case, performing genetic analysis and any accompanying images. Ethical approval was not required for this case report in accordance with the ethics review board of Shinonoi General Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nakamura, H., Ueda, M., Anayama, M. et al. Hilar lymphadenopathy, development of tubulointerstitial nephritis, and dense deposit disease following Pfizer-BioNTech COVID-19 vaccination. CEN Case Rep 12, 287–291 (2023). https://doi.org/10.1007/s13730-022-00762-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-022-00762-7