Abstract
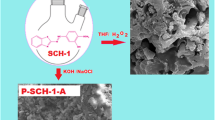
We synthesized the Schiff bases and their polymers, which contain thiophene units. For the synthesis process, first, hydroxynaphthalene di- and triol-derived Schiff bases that are abbreviated as (A1) and (A2) were prepared by a common condensation reaction. Second, their polymers named as B1 and B2 were separately synthesized from the oxidative polycondensation of the Schiff base monomers with (NH4)2S2O8 in an aqueous alkaline medium. The new polymeric structures that are abbreviated as B1-S and B2-S, containing thiophene groups, were synthesized by HBr elimination using B1 and B2 polymers. The characterization processes of the synthesized products were carried out using spectroscopic techniques such as FTIR, UV–Vis, 1H and 13C NMR, TG-DTG analysis, and MALDI-TOF MS. According to the TG analysis results, except for A2, the thermal stabilities of the other compounds were good since they left about 50% residue at 800°C. SEM images of the compounds were recorded at different sizes, and also they were found to be quite different from each other. For example, A1 was like a ball. Additionally, A1, A2, B1, B2, B1-S, and B2-S were tested for their anti-bacterial activities against some bacteria types. Minimum inhibitory concentration (MIC) method was used for the anti-bacterial activity tests. The MIC test results showed that the synthesized polymers could be used as antimicrobial agents for surfaces with P. vulgaris NRRL-B-123 bacteria at lower concentrations such as 15.625 and 31.25 µg/mL.
Graphical abstract


















Similar content being viewed by others
Data availability
The authors do not have permission to share data.
References
Sek D, Iwan A, Jarzabek B, Kaczmarczyk B, Kasperczyk J, Janeczek H, Mazurak Z (2009) Characterization and optical properties of oligoazomethines with triphenylamine moieties exhibiting blue, blue-green and green light. Spectrochim Acta A 72:1–10
Giuseppone N, Fuks G, Lehn JM (2006) Tunable fluorene-based dynamers through constitutional dynamic chemistry. Chem Eur J 12:1723–1735
Grigoras M, Catanescu CO (2004) Imine oligomers and polymers. J Macromol Sci C 44:131–173
Chohan ZH, PraveenM, (2001) Synthesis, characterization, coordination andantibacterial properties of novel asymmetric 1,1′-disubstituted ferrocene-derived Schiff-base ligands and their Co(II), Cu(II) Ni(II) and Zn(II) complexes. Appl Organomet Chem 15:617–625
Pandeya SN, Sriram D, Nath G, De Clercq E (1999) Synthesis, antibacterial, antifungal and anti-hıv activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur J Pharm Sci 9:25–31
Shivarama Holla B, Veerendra B, Shivananda MK, Poojary B (2003) Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 38:759–767
Baran T, Mentes A (2015) Cu(II) and Pd(II) complexes of water soluble O-carboxymethyl chitosan Schiff bases: synthesis, characterization. Int J Biol Macromol 79:542–554
Yılmaz Baran N, Demir HO, Kostekçi S, Saçak M (2015) Poly-2-[(4-methylbenzylidene)amino]phenol: investigation of thermal degradation and antimicrobial properties. J Appl Polym Sci 132:41758
Fernández GJM, del Rio-Portilla F, Quiroz-García B, Toscano RA, Salcedo R (2001) The structures of some ortho-hydroxy Schiff base ligands. J Mol Struct 561:197–207
Ahmed YM, Mohamed GG (2022) New tin(IV) Schiff base complexes: synthesis, characterization and antibacterial investigation, docking and theoretical studies. Inorg Chem Commun 144:109864–109877
Panda L, Duarte-Sierra A (2022) Recent advancements in enhancing antimicrobial activity of plant-derived polyphenols by biochemical means. Horticulturae 8:401–420
Ceramella J, Iacopetta D, Catalano A, Cirillo F, Lappano R, Sinicropi MS (2022) A review on the antimicrobial activity of Schiff bases: data collection and recent studies. Antibiotics 11:191–214
Erturk AG (2020) Synthesis, structural identifications of bioactive two novel Schiff bases. J Mol Struct 1202:127299–127318
Selvi C, Nartop D (2012) Novel polymer anchored Cr(III) Schiff base complexes: synthesis, characterization and antimicrobial properties. Spectrochim Acta A Mol Biomol Spectrosc 95:165–171
Kaya İ, Bilici A, Saçak M (2006) Synthesis, characterization, and antimicrobial properties of oligo-4-[(pyridine-3-yl-methylene) amino] phenol. J Appl Polym Sci 102:3327–3333
Yüksel E, Bilici A, Geçibesler İH, Kaya İ (2020) Synthesis and antioxidant activities of phenolic Schiff base monomers and polymers. Can J Chem 98:151–157
Turan N, Kaya E, Gündüz B, Çolak N, Körkoca H (2012) Synthesis, characterization of poly(e)-3-amino-4-((3-bromophenyl)diazenyl)-1H-pyrazol-5-ol: investigation of antibacterial activity, fluorescence, and optical properties. Fibers Polym 13:415–424
Schiff H (1869) Ueber condensirte Harnstoffe. Justus Liebigs Ann Chem 150:193–200
Berber N, Arslan M (2020) Preparation and characterization of some Schiff base compounds. Adyu J Sci 10:179–188
Fu W, Zou T, Liang X, Wang S, Gao X, Zhang Z, Fang Y (2019) Characterization and thermal performance of microencapsulated sodium thiosulfate pentahydrate as phase change material for thermal energy storage. Sol Energy Mater Sol Cells 193:149–156
Sun Y, Sun G (2002) Synthesis, characterization, and antibacterial activities of novel N-halamine polymer beads prepared by suspension copolymerization. Macromolecules 35:8909–8912
Shaki H (2022) Evaluation of removal of acid dyes using PAM-FeSO4 hybrid polymer as flocculant. Pigment Resin Technol (ahead-of-print)
Sogut EG, Emre D, Bilici A, Kilic NC, Yılmaz S (2022) Porous graphitic carbon nitride nanosheets coated with polyfluorene for removal of malachite green and methylene blue dyes and Cu(II) ions. Mater Chem Phys 290:126523–126535
Kocaeren AA (2016) Synthesis and electrochromic performance of a novel polymer based on an oxidative polymer derived from carbazole and thiophene. J Polym Res 23:66–75
Kocaeren AA (2015) Electrochemical synthesis and electrochromic application of a novel polymer based on carbazole. Org Electron 24:219–226
Kocaeren AA (2016) A new polymer for OLEDs based on carbazole: white, turquoise blue and light orange colors. Int J Plast Technol 20:143–158
Karas M, Bachmann D, Bahr U, Hillenkamp F (1987) Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int J Mass Spectrom Ion Processes 78:53–68
Peterson DS (2007) Matrix-free methods for laser desorption/ionization mass spectrometry. Mass Spectrom Rev 26:19–34
Montaudo G, Montaudo MS, Puglisi C, Samperi F, Spassky N, LeBorgne A, Wisniewski M (1996) Evidence for ester-exchange reactions and cyclic oligomer formation in the ring-opening polymerization of lactide with aluminum complex initiators. Macromolecules 29:6461–6465
Özbülbül A, Mart H, Tunçel M, Serin S (2006) A new soluble Schiff base polymer with a double azomethine group synthesized by oxidative polycondensation. Des Monom Polym 9:169–179
Birol E (2021) Determination of various factors affecting ion generation mechanisms in MALDI-Mass spectrometry. Master of Science Department of Chemistry Supervisor: Prof. Dr. B. Salih 01/2021, p 97
Iwahara K, Honda Y, Watanabe T, Kuwahara M (2000) Polymerization of guaiacol by lignin-degrading manganese peroxidase from Bjerkandera adusta in aqueous organic solvents. Appl Microbiol Biotechnol 54:104–111
Mannu A, Di Pietro ME, Mele A (2020) Band-gap energies of choline chloride and triphenylmethylphosphoniumbromide-based systems. Molecules 25:1495–1506
Gruzdev M, Chervonova U, Kolker A, Fomina N, Zueva E, Vorobeva V, Starichenko D, Korolev A (2021) Dendritic iron(III) carbazole complexes: structural, optical, and magnetic characteristics. Materials 14:5445–5465
Bahçeci DŞ, Demir N, Kocaeren AA (2022) Biological activity and optical sensor properties of green synthesis polymer. Chem Sel 7:e202202096–e202202112
Kocaeren AA (2016) Synthesis and characterization of novel polymers based on carbazole with NaOCl and FeCl3 oxidants. Iran Polym J25:15–24
Bilici A, Dogan F, Yıldırım M, Kaya İ (2012) Tunable multicolor emission in oligo(4-hydroxyquinoline). J Phys Chem C116:19934–19940
Saeed SES, Al-Harbi TM, Alhakimi AN, Abd El-Hady MM (2022) Synthesis and characterization of metal complexes based on aniline derivative Schiff base for antimicrobial applications and UV protection of a modified cotton fabric. Coatings 12:1181–1199
Mighani H (2020) Schiff base polymer: synthesis and characterization. J Polym Res 27:168–186
Romero DB, Nüesch F, Benazzi T, Adès D, Siove A, Zuppiroli L (1997) Electroluminescence from carbazole dimers. Adv Mater 9:1158–1161
Şenol D (2017) Synthesis and characterization of Schiff base polymer dyes containing electron-withdrawing and electron releasing groups. Hacettepe J Biol Chem 45:67–80
Ayyagari MS, Marx KA, Tripathy SK, Akkara JA, Kaplan DL (1995) Controlled free-radical polymerization of phenol derivatives by enzyme-catalyzed reactions in organic solvents. Macromolecules 28:5192–5197
Ünaldı E (2020) Investigation of biological activities and antioxidant properties of sulphonic acid based imine compounds. Çanakkale Onsekiz Mart University Graduate School of Natural and Applied Sciences Master of Science Thesis in Biology Science Advisor: Assist. Prof. Dr. N. Demir 01/15/2020, p 53
Baran NY, Saçak M (2018) Preparation of highly thermally stable and conductive Schiff base polymer: molecular weight monitoring and investigation of antimicrobial properties. J Mol Struct 1163:22–32
Issenberg HD (1998) Essential procedures for clinical microbiology. American Society for Clinical Microbiology, Washingtoon DC, p 126
Kaya E, Erdoğan E, Bursal E, Canpolat E (2021) Synthesis, characterization, optical, morphological, and antioxidant properties of oligo(2-ethoxy-6-(((2-hydroxyphenyl) imino)methyl) phenol) obtained by oxidative polycondensation. Iran Polym J 30:285–295
Acknowledgements
We would like to thank Dr. Neslihan DEMİR (Çanakkale Onsekiz Mart University, Faculty of Sciences and Arts, Department of Biology, Çanakkale¸ Turkey) for her help on anti-microbial tests. Additionally, the authors thank the Çanakkale Onekiz Mart University Scientific Research Projects Coordination Unit (Project No.: FYL-2022-4007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kocaeren, A.A., Karatağ, E. Thiophene-containing Schiff base polymers: synthesis, characterization, and anti-bacterial properties. Iran Polym J 32, 1405–1419 (2023). https://doi.org/10.1007/s13726-023-01211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01211-7