Abstract
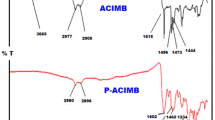
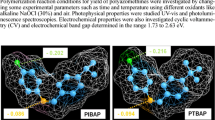
In this study, Schiff bases (SCH-1 and SCH-2) were synthesized from the condensation reaction of 2-aminobenzothiazole with 3-hydroxy-4-methoxybenzaldehyde and 3-hydroxy-4-ethoxybenzaldehyde. Poly(phenoxy-imine)s were synthesized from Schiff bases via oxidative polycondensation by NaOCl (6–14% aqueous solution) as oxidant in alkaline medium and H2O2 (35% aqueous solution) as oxidant in THF medium. The structures characterizations of Schiff bases and poly(phenoxy-imine)s were confirmed by FT-IR, 1H and 13C NMR, CV, UV–Vis and TGA analyses. Limit oxygen index (LOI) and the heat resistance index (THRI) temperature were determined from thermogravimetric measurements of compounds. P-SCH-2-A showed the highest LOI as 45.50 with self-extinguishing according to other polymers (18–30). The optical band gap of P-SCH-2-A was determined to be 1.99 eV. Additionally, the optical band gap energy of compounds were calculated by using the Tauc method. According to the Tauc method, the optical band gap (Eg) value of P-SCH-2-A was calculated to be 2.20 eV. The glass transition temperatures (Tg) and surface properties of poly(phenoxy-imine)s were determined from DSC and SEM analyses, respectively. The weight average molecular weight (Mw) values of P-SCH-1-O, P-SCH-2-O, P-SCH-1-A and P-SCH-2-A were calculated to be 7400, 8400, 3100 and 19,100 g mol−1 from gel permeation chromatography (GPC) measurements.
Graphical abstract












Similar content being viewed by others
References
Ke H, ZhanFg Q, Zhang X, Cheng G, Sun Y, Li J, Cheng H (2021) Hydroquinone-based conjugated Schiff base polymer as anode material for lithium ion batteries. Mater Lett 286:129235. https://doi.org/10.1016/j.matlet.2020.129235
Wei Z, Lü X, Wang W, Mele G, Jiang Z (2023) Excellent removal performance of 4,4’-biphenyldicarboxaldehyde m-phenylenediamine Schiff base magnetic polymer towards phenanthrene and 9-phenanthrol: experimental, modeling and DFT calculations studies. J Hazard Mater 441:129920. https://doi.org/10.1016/j.jhazmat.2022.129920
Sek D, Siwy M, Bijak K, Filapek M, Malecki G, Nowak E, Sanetra J et al (2015) Optical and electrochemical properties of novel thermally stable Schiff bases bearing naphthalene unit. J Electroanal Chem 751:128–136. https://doi.org/10.1016/j.jelechem.2015.05.040
El-Azabawy O, Higazy S, Al-Sabagh A, Abdel-Rahman A et al (2023) Studying the temperature influence on carbon steel in sour petroleum media using facilely-designed Schiff base polymers as corrosion inhibitors. J Mol Struct 1275:134518. https://doi.org/10.1016/j.molstruc.2022.134518
Fei M, Chang Y, Hao C, Shao L, Liu W et al (2023) Baoming Zhao, Jinwen Zhang, Highly engineerable Schiff base polymer matrix with facile fiber composite manufacturability and hydrothermal recyclability. Compos B 248:110366. https://doi.org/10.1016/j.compositesb.2022.110366
El-Bindary A, El-Sonbati A, Diab M, Ghoneim M, Serag L (2016) Polymeric complexes: LXII. Coordination chemistry of supramolecular Schiff base polymer complexes-a review. J Mol Liq 216:318–329. https://doi.org/10.1016/j.molliq.2015.12.113
Yılmaz Baran N, Baran T, Mentes A et al (2018) Highly effective and recoverable Pd(II) catalyst immobilized on thermally stable Schiff base polymer containing phenol group: production, characterization and application in Suzuki coupling reactions. J Organomet Chem 866:87–94. https://doi.org/10.1016/j.jorganchem.2018.04.022
Arafat M, Abdel-Latif E, El-Taweel F et al (2022) Synthesis and biological assessment of new benzothiazolopyridine and benzothiazolyl-triazole derivatives as antioxidant and antibacterial agents. Bull Chem Soc Ethiop 36:451–463. https://doi.org/10.4314/bcse.v36i2.17
Sulthana S, Pandian P (2019) A review on indole and benzothiazole derivatives its importance. J Drug Deliv Ther 9:505–509. https://doi.org/10.22270/jddt.v9i1-s.2358
Hamzah M, Jebur I, Ahmed A (2018) Synthesis, characterization and biological activity evaluation of some new azo derivatives from 2-amino benzothiazole and their derivatives. Kirkuk Univ J Sci Stud (KUJSS) 13:212–227. https://doi.org/10.32894/kujss.2018.143033
Choudhary S, Kalra N, Jeyabalan D (2018) Synthesis, characterization & pharmacological evaluation of some newer benzothiazole derivatives. Indian J Pharm Biol Res (IJPBR) 6:31–36. https://doi.org/10.30750/ijpbr.6.2.6
Ballari M, Cano N, Lopez A et al (2017) Green synthesis of potential antifungal agents: 2-benzyl substituted thiobenzoazoles. J Agric Food Chem 65:10325–10331. https://doi.org/10.1021/acs.jafc.7b04130
Yu X, Yin Q, Zhang Z et al (2019) Synthesis of 2-substituted benzothiazoles via the Brønsted acid catalyzed cyclization of 2-amino thiophenols with nitriles. Tetrahedron Lett 60:1964–1966. https://doi.org/10.1016/j.tetlet.2019.06.039
Karaer Yağmur H, Kaya İ, Aydın H (2020) Synthesis, characterization, thermal and electrochemical features of poly (phenoxyimine)s containing pyridine and pyrimidine units. J Polym Res 27:356. https://doi.org/10.1007/s10965-020-02297-w
Yıldırım M, Kaya İ, Aydın A (2012) Synthesis and characterization of iminothiazole bearing polyphenol with adjustable white-yellow photoluminescence color. Synth Met 162:2443–2450. https://doi.org/10.1016/j.synthmet.2012.11.019
Kaya İ, Püskül T, Karaer Yağmur H (2022) Synthesis and characterization and some properties of conjugated imine bonding polymers containing pyridine and vinyl units. J Polym Res 29:139. https://doi.org/10.1007/s10965-022-02992-w
Yılmaz Baran N, Saçak M (2018) Preparation of highly thermally stable and conductive Schiff base polymer: molecular weight monitoring and investigation of antimicrobial properties. J Mol Struct 1163:22–32. https://doi.org/10.1016/j.molstruc.2018.02.088
Kaya İ, Cihangiroğlu N (2004) Synthesis, characterization and anti-microbial activity of oligo-N-2-aminopyridinylsalicylaldimine and some oligomer-metal complexes. J Polym Res 11:37–42. https://doi.org/10.1023/B:JPOL.0000021746.50347.34
Özbülbül A, Mart H, Tunçel M, Serin S (2006) A new soluble Schiff base polymer with a double azomethine group synthesized by oxidative polycondensation. Des Monomers Polym 9:169–179. https://doi.org/10.1163/156855506776382655
Kaya İ, Yıldırım M, Avcı A, Kamacı M (2011) Synthesis and thermal characterization of novel poly(azomethine-urethane)s, derived from azomethine containing phenol and polyphenol species. Macromol Res 19:286–293. https://doi.org/10.1007/s13233-011-0306-1
Kaya İ, Yıldırım M, Aydın A, Şenol D (2010) Synthesis and characterization of fluorescent graft fluorene-co-polyphenol derivatives: the effect of substituent on solubility, thermal stability, conductivity, optical and electrochemical properties. React Funct Polym 70:815–826. https://doi.org/10.1016/j.reactfunctpolym.2010.07.013
Tezel RN, Kaya İ (2020) Thiophene substituted phenothiazine polymers: design, synthesis and characterization. Arab J Chem 13:3123–3136. https://doi.org/10.1016/j.arabjc.2018.09.004
Karaer H, Kaya İ, Aydın H (2017) Synthesis, characterization, thermal and electrochemical properties of imine polymers containing pyridine and pyrimidine units. Polimery 62:170–180. https://doi.org/10.14314/polimery.2017.170
Yıldırım M, Kaya İ (2012) A comparative study of aminothiazole-based polymers synthesized by chemical oxidative polymerization. Synth Met 162:436–443. https://doi.org/10.1016/j.synthmet.2012.01.010
Nishat N, Khan S, Rasool R, Parveen S (2011) Synthesis, spectral characterization and biocidal activity of thermally stable polymeric Schiff base and its polymer metal complexes. J Inorg Organomet Polym 21:673–681. https://doi.org/10.1007/s10904-011-9457-y
Maruthapandi M, Kumar V, Gedanken A (2018) Carbon dot initiated synthesis of poly(4,4′- diaminodiphenylmethane) and its methylene blue adsorption. ACS Omega 3:7061–7068. https://doi.org/10.1021/acsomega.8b00304
Xu Z, Kong L, Wang Y et al (2023) Tuning band gap, color switching, optical contrast, and redox stability in solution-processable BDT-based electrochromic materials. Org Electron 54:94–103. https://doi.org/10.1016/j.orgel.2017.12.014
Abd-Elnaiem A, Salman O, Hakamy A, Hussein S (2022) Mechanical characteristics and thermal stability of hybrid epoxy and acrylic polymer coating/nanoclay of various thicknesses. J Inorg Organomet Polym Mater 32:2094–2102. https://doi.org/10.1007/s10904-022-02270-8
Sengottuvelu D, Athianna M, Siddeswaran A (2021) High temperature stable conjugated polyazomethines containing naphthalene moiety: synthesis, characterization, optical, electrical and thermal properties. Spectrochim Acta Part A Mol Biomol Spectrosc 246:118989. https://doi.org/10.1016/j.saa.2020.118989
Liu S, Zhang X, Bu M, Lei C (2022) Properties tailoring of biobased epoxy resins by regulating the degree of polymerization of oligomers. Eur Polym J 173:111253. https://doi.org/10.1016/j.eurpolymj.2022.111253
Liu B, Essawy H, Deng S et al (2023) High performance bio-based gelatinized starch-furanic resin derived foam reinforced by microcrystalline cellulose. Ind Crops Prod 194:116282. https://doi.org/10.1016/j.indcrop.2023.116282
Novozhilov V, Joseph P, Ishiko K et al (2011) Polymer combustion as a basis for hybrid propulsion: a comprehensive review and new numerical approaches. Energies 4:1779–1839. https://doi.org/10.3390/en4101779
Silva-Santos M, Oliveira M, Giacomin A et al (2017) Flammability on textile of flight crew professional clothing. IOP Conf Ser Mater Sci Eng 254:052006
Satdive A, Mestry S, Borse P et al (2020) Phosphorus and silicon containing amino curing agent for epoxy resin. Iran Polym J 29:433–443. https://doi.org/10.1007/s13726-020-00808-6
İçduygu M, Asiltürk M, Yalçınkaya M et al (2019) Three-dimensional nano-morphology of carbon nanotube/epoxy filled poly(methylmethacrylate) microcapsules. Materials 12:1–26. https://doi.org/10.3390/ma12091387
Wang J, Wang F, Gao Z et al (2016) Flame retardant medium-density fiberboard with expanded vermiculite. Bioresources 11:6940–6947. https://doi.org/10.15376/biores.11.3.6940-6947
Amin P, Ketuly K, Saeed S et al (2021) Synthesis, spectroscopic, electrochemical and photophysical properties of high band gap polymers for potential applications in semi-transparent solar cells. BMC Chem 15:1–15. https://doi.org/10.1186/s13065-021-00751-4
Colladet K, Nicolas M, Goris L et al (2004) Low-band gap polymers for photovoltaic applications. Thin Solid Films 451–452:7–11. https://doi.org/10.1016/j.tsf.2003.10.085
Ajayaghosh A (2003) Donor–acceptor type low band gap polymers: polysquaraines and related systems. Chem Soc Rev 32:181–191. https://doi.org/10.1039/b204251g
Koyuncu S, Kaya İ, Baycan Koyuncu F, Ozdemir E (2009) Electrochemical, optical and electrochromic properties of imine polymers containing thiophene and carbazole units. Synth Met 159:1034–1042. https://doi.org/10.1016/j.synthmet.2009.01.024
Ponnappa S, Arumugam S, Spratt H, Manzhos S et al (2017) A comparative study of electrochemical, optical properties and electropolymerization behavior of thiophene- and furan-substituted diketopyrrolopyrrole. J Mater Res 32:810–821. https://doi.org/10.1557/jmr.2017.26
Grigoras M, Antonoaia N (2005) Synthesis and characterization of some carbazole-based imine polymers. Eur Polym J 41:1079–1089. https://doi.org/10.1016/j.eurpolymj.2004.11.019
Mir F (2014) Transparent wide band gap crystals follow indirect allowed transition and bipolaron hopping mechanism. Results Phys 4:103–104. https://doi.org/10.1016/j.rinp.2014.06.001
Jubu P, Obaseki O, Nathan-Abutu A et al (2022) Dispensability of the conventional Tauch’s plot for accurate bandgap determination from UV-Vis optical diffuse reflectance data. Results Opt 9:100273. https://doi.org/10.1016/j.rio.2022.100273
Cimrov V, Ulbricht C, Dzhabarov V et al (2014) New electroluminescent carbazole-containing conjugated polymer: synthesis, photophysics, and electroluminescence. Polymer 55:6220–6226. https://doi.org/10.1016/j.polymer.2014.10.015
Misra A, Kumar P, Srivastava R et al (2005) Electrochemical and optical studies of conjugated polymers for three primary colours. Indian J Pure Appl Phys 43:921–925
Boborodea A, O’Donohue S, Brookes A (2021) A new GPC/TREF/Lin ELSD instrument to determine the molecular weight distribution and chemical composition: application to recycled polymers. Int J Polym Anal Charact 26:721–734. https://doi.org/10.1080/1023666X.2021.1971836
Viéville J, Tanty M, Delsuc M (2011) Polydispersity index of polymers revealed by DOSY NMR. J Magn Reson 212:169–173. https://doi.org/10.1016/j.jmr.2011.06.020
Shrivastava A (2018) Polymerization. Introduction to plastics engineering plastics design library, pp 17–48. ISBN: 9780323396196
Acknowledgements
The authors thank Canakkale Onsekiz Mart University scientific research project commission for support with the project number (Project Nu.: FYL-2021-3574).
Author information
Authors and Affiliations
Contributions
İK: Supervision, methodology, conceptualization, data curation, writing-review & editing, funding acquisition, resources, project administration. ED: Data curation, methodology, writing-review & editing, original draft, validation, conceptualization. HKY: Writing-review & editing, original draft, conceptualization, data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaya, İ., Dinçer, E. & Yağmur, H.K. Synthesis, characterization, thermal and electrochemical properties of poly (phenoxy-imine)s containing benzothiazole unit. Polym. Bull. (2023). https://doi.org/10.1007/s00289-023-05062-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-023-05062-3