Abstract
The role of iron(III) oxide (Fe2O3) in the cross-linking process, structure and functional properties of chloroprene and butadiene rubber (CR/BR) blends was studied. The unconventional elastomeric blends containing chloroprene and butadiene rubbers of different mass ratios, cross-linked with iron(III) oxide, have been studied. It has found that the iron(III) oxide could be used as a cross-linking agent in CR/BR blend and the curing degree depends largely on the composition of the blends. These results indicate that the curing degree of the blends is increased with increasing amount of chloroprene rubber. It is very interesting to note that the addition of butadiene rubber to chloroprene rubber improved the mechanical properties of the resulting vulcanizates. The resulting CR/BR/Fe2O3 vulcanizates were characterized by good mechanical properties. Surprisingly, the selected vulcanizates containing chloroprene and butadiene rubbers had a 40% greater tensile strength compared to vulcanizates containing only chloroprene rubber. The amount of iron(III) oxide as the curing agent slightly affected the curing degree and mechanical properties of the resulting rubber materials. This should probably be linked to the interelastomer reaction between the chloroprene and butadiene rubbers occurring in the presence of iron(III) oxide at an elevated temperature. Interelastomer reactions between both elastomers may lead to improved homogeneity and miscibility of the test systems. Additionally, it has been found that the CR/BR blends cross-linked with Fe2O3 were characterized by a high flame resistance. The undoubtable advantages of the proposed technology are its simplicity, low cost and incombustibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Butadiene rubber (BR) is obtained by the addition polymerization of 1,3-butadiene. In the polymerization process of butadiene, three different geometric isomers are formed in the polymer chain (trans-1.4, cis-1.4, vinyl-1.2) (Scheme 1). The BR of cis-1.4 content 90% or above is produced by an anionic coordination polymerization using Ziegler–Natta catalysts (cobalt, nickel or titanium catalysts). This stereoregular BR is characterized by a high degree of regularity and exhibits a low glass transition temperature value (− 105 °C). The double bonds in BR macromolecules are highly reactive and react with sulfur, peroxides and various other cross-linking agents to give a cured polymer. The vulcanizates of BR have superior tensile strength, low hysteresis loss, good wear resistance, low rolling resistance, and for these reasons about 70% of the total production of BR is used in the tire industry. Butadiene rubber is commonly blended with other rubbers instead of being used alone [1,2,3,4,5,6,7].
The other ingredient of studied composites—chloroprene rubber (CR)—belongs to the group of special elastomers because of its unique characteristics (polar chain structure, good oil resistance and resistance to flame and ozone) [8, 9]. On a large scale, the polymerization of chloroprene is carried out during the free radical emulsion polymerization. CR preparation is a multi-step process. The standard chloroprene rubber has a highly regular structure and mainly consists of trans-units. The presence of cis-units and 1.2- or 3.4-structures is also observed (Scheme 2). These CR structures are combined in sequential isomers derived from head-to-tail, head-to-head and tail-to-tail addition [10, 11].
The chemical, physical or rheological properties of chloroprene rubber largely depend on changes in the molecular structure, especially the trans/cis ratio and long chain branching. High concentrations of the same repeated trans-1.4-units result in a high degree of polymer crystallinity. Crystallization is completely reversible by the heating or dynamic stress. A chloroprene rubber containing multiple trans-1.4-units is a hard material with a little elasticity. When CR is obtained at higher temperatures, it consists of larger amounts of irregular structures and crystallizes slowly. Due to the presence of substantially inactive >C=C< bonds compared to other diene rubbers, chloroprene rubber cannot be cross-linked with sulfur. Therefore, CR is conventionally cured with zinc oxide (ZnO) in the presence of magnesium oxide (MgO) [8, 12]. Chloroprene rubber can be effectively cross-linked with other metal oxides, such as iron(III) oxide (Fe2O3) or iron(II,III) oxide (Fe3O4) [13]. The curing of chloroprene rubber with tin(II) oxide (SnO) is also possible and the resulting CR is characterized by better mechanical properties than the conventionally cross-linked CR [14]. Satisfactory properties of the vulcanizates have been achieved by cross-linking the CR with copper(I) oxide (Cu2O) or copper(II) oxide (CuO) [15]. Vulcanizates of CR have good resistance to tear, flame, aging, non-polar solvents, oil or ozone. These products are also characterized by good mechanical properties and good adhesion to other materials, such as textiles or metals [16,17,18]. Owing to the presence of halogen in the rubber macromolecules, rubber goods containing CR resist burning inherently better than any other hydrocarbon rubbers [19].
Chloroprene rubber has recently been combined with other elastomers with the view to achieving special properties of either the CR-based compounds or compounds based on second ingredients. The purpose of blending two or several elastomer types is to improve processing and mechanical properties and reduce the compounding cost. Blending of elastomers can be practically carried out in several different ways, for example, latex, solution blending and mixing of solid rubbers on mills, in internal mixers or during continuous mixing [20].
Only few communications concerning CR blends with butadiene rubber have appeared in the literature. Mingyi et al. [21] studied the structure and properties of chloroprene, butadiene rubber and styrene–butadiene–styrene (CR/BR/SBS) copolymer blends. The addition of styrene–butadiene–styrene copolymer to thermodynamically immiscible CR/BR blends improves its homogeneity and mechanical properties and increases the cross-linking density of vulcanizates [21]. BR displays low temperature resistance and high electrical insulation properties due to its regular molecular structure, flexible molecular chain and the absence of substituted groups in the main chain. The addition of BR to CR improves low temperature resistance and electrical insulation properties of CR [9]. The elastomeric blend containing chloroprene and butadiene rubbers cross-linked with copper(II) oxide (Cu2O) or copper(II) oxide (CuO) was also studied [22]. It was found that CR/BR/Cu2O and CR/BR/CuO vulcanizates are characterized by a high degree of cross-linking, which is increasing with the amount of chloroprene rubber in the blends. The cross-linking reactions are accompanied by the formation of bonds between the chloroprene and butadiene rubber. The cross-linking of CR/BR/Cu2O or CR/BR/CuO blends follows a cationic mechanism, using Lewis acid, copper(I) chloride (CuCl) or copper(II) chloride (CuCl2) which is in situ generated [22]. It is worth noting that the performance of elastomeric blends depends on the ability of all components miscibility with each other, because blends with good miscibility and phase adhesion exhibit better performance than those weakly blended [20].
The aim of our study was to analyze the role of iron(III) oxide on the cross-linking process of CR/BR blends and the morphology and properties of the new resulting products. The use of iron(III) oxide as a curing agent for CR/BR blends is a novel idea [23]. We assumed that the blends prepared and cross-linked according to our method would be characterized by increased resistance to burning. Certainly, this method of manufacturing non-flammable rubber materials is non-standard and has not been reported in the literature.
Experimental
Materials
Chloroprene rubber (CR) (Baypren®216 MV from Lanxess GmbH, Dormagen, Germany), and butadiene rubber (BR) (SYNTECA®44 from Synthos S.A., Poland) were used as elastomers. Iron(III) oxide (Fe2O3) (Sigma-Aldrich Chemical Co., Germany) with a density of 5.2 g/cm3 was used as a curing agent. Stearic acid (Chemical Worldwide Business Sp. z o. o., Poland) was used as a plasticizer; silica (Ultrasil 7000 GR, Evonik Industries AG, Germany), with a specific surface area of 175 m2/g and a density of 0.27 g/cm3, was used as a filler and china clay (POCh S.A., Poland), with a density of 2.6 g/cm3, was used as a filler.
Method testing
CR/BR/Fe2O3 blends were prepared in a conventional way using a standard laboratory two-roll mill (T = 40–50 °C, t = 10 min). The blends were then pressed in an electrically heated hydraulic press at 160 °C. Vulcametric measurements were determined by a WG-02 rheometer, at 160 °C, according to PN-ISO 3417:2004. Curing degree was determined on the basis of vulcametric measurements, equilibrium swelling in toluene or heptane, extraction with boiling acetone and the Mooney–Rivlin elasticity constants (2C1, 2C2) determined from the Mooney–Rivlin equation:
where P is the deformation force at λ (kg), λ is the deformation (λ = l/l0), l is the measuring section of the sample loaded with P (cm), l0 is the measuring section of the unloaded sample (cm), A0 is the cross-sectional area of the unloaded sample (cm2), 2C1 is the first elastic constant (kg/cm2), and 2C2 is the second elastic constant (kg/cm2).
The network structure and cross-linking degree of the cured CR/BR blends were characterized by FTIR spectroscopy. FTIR spectra were made in transmission mode using a Bio-Rad 175C IR spectrophotometer. Test samples having a thickness of 0.03–0.05 mm were prepared using a hydraulic press at a temperature of 70 °C.
Differential scanning calorimetry (DSC) was used to characterize the changes during the heating of CR/BR blends. Samples were scaled in aluminum pans, and their thermal properties were measured using a DSC 204 Netzsch apparatus. The measurements were carried out at a temperature range of (−)120–200 °C. Thermograms were recorded at a heating rate of 10 K/min.
The morphology of the CR/BR/Fe2O3 vulcanizates was investigated with a scanning electron microscopy (SEM) by Hitachi S-4700 (Japan) with the ThermoNoran Energy Dispersive Spectrometer (EDS) micro-analysis adapter. The samples were sputter coated with carbon for 4 min under high vacuum before an examination. The magnification of image was 5000.
Mechanical properties: stress-at-elongation of 100%, 200%, 300% (Se100,Se200,Se300), tensile strength (TSb) and elongation-at-break (Eb) were determined using a Zwick machine conforming to PN-ISO 37:2007. Based on the aging index, changes of mechanical properties caused by thermo-oxidative aging were evaluated. Aging index (K) was determined in Eq. (2):
where \({\text{TS}}_{{\text{b}}}^{\prime }\) is the tensile strength after thermo-oxidative aging (MPa), TSb is the tensile strength before thermo-oxidative aging (MPa), \(E_{{\text{b}}}^{\prime }\) is the elongation-at-break after thermo-oxidative aging (%), and Eb is the elongation-at-break before thermo-oxidative aging (%).
Flammability of the blends was determined by the oxygen index method using a Fire Testing Technology apparatus. Samples sizes were 50 × 10 × 4 mm. Constant nitrogen flow rate was 400 l/h. We selected an oxygen concentration that enabled the sample to be completely burned within 180 ± 10 s. A sample tip was ignited for 5 s by means of a gas burner supplied with a propane–butane mixture. The oxygen index (OI) was calculated as the percentage of oxygen and nitrogen volume in the mixture:
where O2 is the oxygen flow rate (l/h), and N2 is the nitrogen flow rate (l/h).
Flammability in the air was determined using the same samples as those in the oxygen index method. A sample in vertical position was ignited with a gaseous burner for 5 s and its combustion time (tc) was measured.
Results and discussion
Cross-linking of CR/BR blends with iron(III) oxide
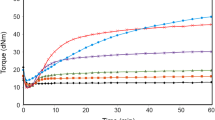
Rubber products acquire valuable properties in the final cycle of processing only, i.e., during the curing process, which has important consequences to the network structure and properties [24]. Therefore, in first step of the study, the effects of elastomer mass ratio in the CR/BR blends cured with iron(III) oxide (Fe2O3) on cross-linking kinetics and the selected properties were assessed (Table 1). For this purpose, different molar ratios of CR and BR and of iron(III) oxide in the amount of 3 phr were used. We found that the CR/BR blends could be cross-linked with iron(III) oxide and that curing degree depended largely on the composition of the blends. In terms of vulcametric results, the sample containing CR only (CR/BR: 100/0 by wt) was characterized by the shortest scorch time (τ02 = 3.2 min). The combination of butadiene rubber with chloroprene rubber led to a prolonged scorching time. The scorching time of the CR/BR (80/20 by wt) blend was 6.9 min, whereas the cure time (τ90) of the sample containing CR only (CR/BR: 100/0 by wt) was 10.4 min. However, in case of the CR/BR (75/25 or 80/20 by wt) blends, we observed a shorter cure time (~ 9 min). The increase in the amount of BR in CR/BR blends prolonged the cure time. The τ90 value of the CR/BR (20/80 by wt) blend exceeded 24 min (Table 1; Fig. 3). The minimum vulcametric torque did not depend significantly on elastomer proportionality in the CR/BR blends. The Lmin for all the blends was similar at ~ 18 dN m. However, in the case of the mix containing BR only (CR/BR: 0/100 by wt), the Lmin value was the highest (23 dN m), which proves that this sample revealed the highest viscosity.
We studied the effect of iron(III) oxide on the curing degree of the CR/BR blends. In the case of CR (CR/BR: 100/0 by wt), the torque increase after 15 min of heating (ΔL15) was 65.4 dN m. The addition of BR to CR decreased the curing degree. The ΔL15 of the CR/BR (80/20 by wt) blend was 43.4 dN m, while the CR/BR (75/25 or 40/60 by wt) blends were characterized by a ΔL15 of 38.9 or 30.5 dN m, respectively. These results indicate that the curing degree decreased with increasing amount of BR in the blends we studied. For the CR/BR (20/80 by wt) blend, only a slight torque increase after 40 min of heating was observed. Additionally, the heating of a mix containing BR only (CR/BR: 0/100 by wt) in the presence of iron(III) oxide did not lead to its cross-linking. In this case, the torque increase after 40 min of heating was slightly above 0 dN m. This proves that BR was not susceptible to curing with Fe2O3 (Table 1; Fig. 1).
FTIR spectra of the CR/BR/Fe2O3 blends before and after cross-linking showed that the relative absorption intensities in the range of 1600–1700 cm−1 (>C=C< group) changed during the heating of CR/BR in the presence of Fe2O3 (Fig. 2). A clear change in relative absorption intensity at 965 cm−1 (>C=C<trans in 1.4-trans-units) and a decrease in relative absorption intensity at 670 cm−1 (>C=C<cis in 1.4-cis-units) were also observed. In addition, the spectrum of CR/BR heated with iron(III) oxide showed a new intensity at 3440 cm−1 characteristic for the hydroxyl group. This most probably means that during the heating of CR/BR blends with Fe2O3, a cis–trans isomerization occurred under the influence of iron(III) chloride (FeCl3), which was generated in situ. This may indicate to interelastomer reactions between the chloroprene and butadiene rubbers. However, the detailed studies of the cross-linking mechanism of CR/BR blends with iron(III) oxide are still continued. A proposed cross-linking mechanism of CR/BR/Fe2O3 composites is shown in Scheme 3.
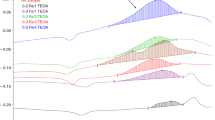
Interelastomer reactions between CR and BR may lead to improved homogeneity and miscibility of the test systems. This was confirmed by DSC analysis and the glass transition temperature (Tg) determined using this method. The Tg depends on the structure and mobility of its chain segments. The immiscibility of elastomers is traditionally signaled by the appearance of two unbroadened glass transition temperatures which remain unchanged compared to pure components of the blends. In the case of partially compatible blends, the two glass transition temperatures were different from the corresponding Tgs of the components [25, 26]. Figure 3 shows the DSC curves of the cured CR/BR blends. The presence of two Tg points was one of the distinctive features of elastomeric blends and each one of the Tg values was associated with the corresponding phases of the blend, i.e. CR and BR. However, small changes in the values of both Tg were observed, which may indicate a partial miscibility of the CR phase and the BR phase. This phenomenon has reduced the temperature interval between two glass transitions. The DSC curve of pure CR cured with iron(III) oxide shows the peak at 45 °C resulting from the presence of crystalline phase typical to CR. It is interesting to notice that the incorporation of BR into CR and further cross-linking of this blend with Fe2O3 led to loss of the peak corresponding to the crystalline phase of CR.
The partial miscibility of CR and BR in the presence of iron(III) oxide was confirmed also by the SEM images. The SEM photographs of the fracture surfaces of CR/BR (75/25 by wt) blend cured with iron(III) oxide (3 phr) are shown in Fig. 4. The rough fracture surface morphology was observed. These results indicate that the compatibility of the CR/BR/Fe2O3 blend was limited, and the incompatibility of the CR/BR/Fe2O3 blend was due to the difference in polarity between both elastomers (CR and BR). However, there was no visible phase separation in studied blends, which means that the CR/BR/Fe2O3 blend was homogeneous in macrostructure.
Effect of elastomeric ratio of CR/BR/Fe2O3 blends on the properties of vulcanizates
We have found in our study that a mix containing CR only (CR/BR: 100/0 by wt) was characterized by the highest curing degree. For this sample, the equilibrium swelling in toluene was 4.65 mL/mL. The incorporation of BR into CR and a further cross-linking with Fe2O3 led to an increase in equilibrium swelling degree in toluene. In the case of the CR/BR (80/20 or 75/25 by wt) vulcanizates, the \(Q_{{\text{v}}}^{{\text{T}}}\) was 5.97 or 6.56 mL/mL, respectively (Table 2). Samples containing butadiene rubber only completely dissolved in toluene or heptane, which confirm that butadiene rubber cannot be cured in the presence of Fe2O3. This is consistent with previous conclusions. Our study showed that the first elasticity constant determined from the Mooney–Rivlin equation, which is directly proportional to the network density, was similar (2C1 = 2.55 kG/cm2) to that of the cured blends containing from 75 to 100 phr of CR. The CR/BR (40/60 and 20/80 by wt) vulcanizates were characterized by significantly lower 2C1 values. This is an indication of a reduction in the curing degree of these samples.
The mechanical properties of CR/BR/Fe2O3 vulcanizates were studied to analyze the resulting rubber materials. The vulcanizates containing CR only (CR/BR: 100/0 by wt) were characterized by a tensile strength (TSb) of 7.14 MPa and an elongation-at-break (Eb) of 687%. It is also important to note that the addition of BR to CR improved the mechanical properties of the resulting vulcanizates. Surprisingly, the CR/BR (80/20 and 75/25 by wt) vulcanizates had a tensile strength of ~ 10 MPa (Table 2; Fig. 5). This should probably be linked to the interelastomeric reaction between CR and BR occurring in the presence of Fe2O3 at an elevated temperature (160 °C). However, further increase in the amount of butadiene rubber in the CR/BR vulcanizates led to a decrease in tensile strength and an increase in elongation-at-break. These results were confirmed by the degree of BR bound with CR, which depends on the amount of individual components in the blends (Table 2). The greatest value of BR bound with CR (68%) in interelastomeric network is observed in the case of CR/BR (75/25 by wt) vulcanizates. The smaller amount of BR in the studied blends led to interelastomer reactions proceeding with a lower yield. In the case of the CR/BR (80/20 by wt) vulcanizates, the degree of BR bound with CR was only 24%.
To assess the resistance of CR/BR vulcanizates to thermo-oxidative aging, the samples were kept at 70 °C for 7 days. We found that the resulting cured products were characterized by a low resistance to thermo-oxidative aging (Table 2; Fig. 5). We observed that in the case of cured CR/BR (75/25 by wt), the tensile strength decreased from 10.2 to 2.8 MPa and the aging index was only 0.08. The average tensile strength of all samples was 2.32 ± 0.53 MPa, regardless of the elastomeric content. These results are not unexpected, as standard CR vulcanizates need protection by antioxidants against thermal aging and by anti-ozonants to improve ozone resistance. The use of these ingredients also improves flex-fatigue resistance [1].
Effect of Fe2O3 amount on the cross-linking of CR/BR blends and the properties of vulcanizates
The estimation of the amount of curing agent incorporated into elastomeric blends is very important from the technological and economic points of view. Therefore, in the next step of our study, several elastomeric blends (CR/BR: 75/25 by wt) with varying amounts of iron(III) oxide (1–5 phr of Fe2O3) were prepared (Table 3). In terms of vulcametric parameters, we found that the amount of iron(III) oxide used as a curing agent affected the scorch time (τ02) of the CR/BR (75/25 by wt) blends. In the case of the CR/BR blend cross-linked with 1 phr of Fe2O3, the longest τ02 (6.5 min) was observed, while the CR/BR blend containing 5 phr of Fe2O3 was characterized by the shortest τ02 (3.5 min). We have also found that in all studied compounds the curing time was in the range of 8–10 min. The amount of Fe2O3 insignificantly affected the curing degree of the CR/BR blends. In all the blends, the torque increase after 15 min of heating was similar (~ 42 dN m). It follows from these results that all studied blends showed comparable curing degrees. The kinetics results were confirmed by the equilibrium swelling degree and the Mooney–Rivlin elasticity constants (Table 3; Fig. 6). The equilibrium swelling degree of the vulcanizates was ∼ 6.3 mL/mL, independent of the Fe2O3 amount used. For all the samples, the same value of the real extract (0.170 mg/mg) was observed. We have also noticed that the amount of Fe2O3 did not affect the first elasticity constant, which was similar for all the samples. The 2C1 value for all the vulcanizates was ∼ 2.44 kG/cm2, which shows a comparable curing degree of the resulting rubber products.
The test of mechanical properties of the resulting CR/BR vulcanizates confirmed that the Fe2O3 amount marginally affected these parameters (Table 3; Fig. 6). For all the products, the stress-at-elongation of 100%, 200% or 300% was in that order ∼ 0.68 MPa, ∼ 1.07 MPa or ∼ 1.41 MPa. The comparable values of these parameters indicated similar stiffness of the studied samples. The tensile strength-at-break was in the range of 9–11 MPa and the elongation-at-break ranged 700–840%. Unfortunately, the resulting rubber materials were characterized by a low resistance to thermo-oxidative aging, as the samples exposed to aging factors showed a significant deterioration of their tensile properties. The tensile strength of the CR/BR vulcanizate containing 2.5 phr of Fe2O3 decreased from 10 to 1.5 MPa after thermo-oxidative aging. The aging index of the products did not exceed 8%, regardless of the amount of the iron(III) oxide.
Flammability and mechanical properties of filled CR/BR/Fe2O3 vulcanizates
Generally, chloroprene rubber can be filled with carbon black or mineral fillers. Active fillers incorporated into CR improve the physical properties, whereas less active or predominantly inactive fillers reduce the compound cost. Therefore, in the next stage of our study, we decided to investigate the effect of active and semi-active fillers on the properties of the resulting vulcanizates. For this purpose, theCR/BR (75/25 by wt) blends cured with 2.5 phr of iron(III) oxide were selected for further analysis. Silica or china clay was used as the filler.
Based on vulcametric measurements, we found that the incorporation of the fillers into theCR/BR/Fe2O3 blends affected their curing degree and the properties of the resulting products (Table 4). The application of an active or a semi-active filler changed the scorch and vulcanization times and the viscosity of the resulting blends. We have also found that the filler did not lead to a significant change of vulcametric torque. These conclusions were also confirmed by the swelling degree. The equilibrium swelling degree in toluene (5.91 or 5.88 mL/mL) determined for the CR/BR/Fe2O3 vulcanizates with silica or china clay decreased compared to unfilled products (6.17 mL/mL), indicating a reduction in the curing degree in the case of filled vulcanizates, regardless of the filler type.
Assessment of the mechanical properties of the resulting rubber materials showed that the use of fillers in the CR/BR/Fe2O3 blends and their subsequent cross-linking had a positive effect (Table 4). The stress-at-elongation of 100%, 200% or 300% definitely assumed higher values for the vulcanizates containing silica (Se100 = 2.29 MPa, Se200 = 3.36 Se300 = 4.59 MPa) than for those with china clay (Se100 = 1.07 MPa, Se200 = 1.66 MPa, Se300 = 2.15 MPa). The presence of silica in CR/BR/Fe2O3 vulcanizates increased the tensile strength to 11.3 MPa, compared to the unfilled compound (TSb = 10.03 MPa). The TSb of the cured CR/BR/Fe2O3/china clay blend was 11.6 MPa. As expected, the filled compounds did not show a high resistance to thermo-oxidative aging. The tensile strength of the silica-containing vulcanizates decreased from 11.3 to 6.58 MPa (before and after thermo-oxidative aging, respectively). Analysis of this stage of the study indicated that the CR/BR/Fe2O3 compounds filled with an active filler (silica) or a semi-active (china clay) led to slight improvements in the strength properties. The presence of the active filler had a comparable effect to the presence of the semi-active filler. This may be related to the nature of chloroprene rubber as an elastomer which does not require the use of a reinforcing filler.
The predominant group of rubber products is flammable materials. Many studies nowadays are therefore aimed at searching for materials that are thermally stable, fire resistant and susceptible to ignition, and release little heat, toxic gases and fumes during their combustion. According to oxygen index (OI), the materials were divided into [27]: flammable materials (OI ≤ 21%), flame-retardant materials (OI = 21–28%), and non-flammable materials (OI ≥ 28%). Notably, cured chloroprene rubber is inherently flame retardant [1]. The OI of conventionally cross-linked CR (e.g., with zinc oxide and magnesium oxide) is 26% [28]. However, the flame resistance of the CR vulcanizates is not sufficient and for many applications further improvement is needed. To achieve a higher flammability of CR vulcanizates, aluminum trihydrate, zinc borate or antimony trioxide is used [1]. In our study, we have decided to investigate the flammability of the CR/BR/Fe2O3 products. The flammability was determined by both the OI and the combustion time in the air (tc). The OI determined for the CR/BR/Fe2O3 vulcanizates filled with silica or china clay was similar (OI > 36%). During the combustion of the samples filled with silica, large amounts of soot and smoke were observed. The flammability in the air using identical samples as in the case of the OI method was tested. All the vulcanizates that combusted in the air were extinguished. The combustion time for all the samples did not exceed 7 s.
Notably, the OI of the conventionally cured chloroprene rubber was 26%, which made it a flame-retardant material. The OI of the conventionally cured (with sulfur) butadiene rubber was only 17%, which indicated an extremely flammable material (Fig. 7). When both elastomers were mixed and cross-linked in our unconventional way (using Fe2O3), new CR/BR/Fe2O3 products with significantly higher OI (> 37%) were obtained. This confirms that the rubber materials proposed in our study are non-flammable products. This significant fire resistance of the CR/BR/Fe2O3 vulcanizates is probably the result of interelastomer reactions between chloroprene and butadiene rubbers during their cross-linking with iron(III) oxide.
Conclusion
Chloroprene and butadiene rubber blends may be cured with iron(III) oxide and the curing degree of these compounds depends on their composition, and in particular on the molar ratio of both elastomers. The curing degree increased with chloroprene rubber content in the CR/BR blends. The resulting CR/BR/Fe2O3 vulcanizates were characterized by good mechanical properties. The amount of iron(III) oxide as the curing agent slightly affected the curing degree and mechanical properties of the resulting rubber materials. The cross-linking reactions were accompanied by interelastomer bounding of chloroprene rubber and butadiene rubber. The presence of active or semi-active fillers in the CR/BR/Fe2O3 vulcanizates led to an insignificant improvement in the strength properties. The type of filler in a specific application must be selected carefully, depending on the requirements of its final use. However, the most important property of the rubber products we obtained, both unfilled and filled with active or semi-active fillers, was the high fire resistance. The vulcanizates proposed here can be used in the manufacture of different technical rubber goods. Simple technology and low manufacturing cost of the incombustible materials are the most important advantages of the proposed CR/BR/Fe2O3 products. Rubber goods obtained in the proposed way can, for example, be used as conveyor belts, wire and cable jacketing, coated fabrics, inflatables, extrusions and many other articles.
References
Chatarsa C, Prasassarakich P, Rempel GL, Hinchiranan N (2015) The influence of Ni/Nd-based Zigler–Natta catalyst on microstructure configurations and properties of butadiene rubber. J Appl Polym Sci 41834:1–9
Takuo S (2016) Industrial synthetic method of the rubbers. 1. Butadiene rubber. Int Polym Sci Technol 1:49–54
Malas A, Pal P, Das CK (2014) Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mater Des 55:664–673
Kim M, Kim G (2013) Effect of processing steps and organoclay content in butadiene rubber/organoclay nanocomposites. J Appl Polym Sci 129:3512–3517
Markovic G, Veljkovic O, Marinovic-Cincovic A, Jovanovic V, Samarzija-Jovanovic S, Budinski-Simendic J (2013) Composites based on waste rubber power and rubber blends: BR/CSM. Compos Part B Eng 45:178–184
Marzocca AJ, Garraza ALR, Sorichetti P, Mosca HO (2010) Cure kinetics and swelling behaviour in polybutadiene rubber. Polym Test 29:477–482
Witinuntakit T, Poompradub S (2015) Preparation of dichlorocarbene modified butadiene rubber and its thermal properties. Macromol Symp 354:111–117
Musch R, Magg H (2008) Polychloroprene rubber. In: Klingender RC (ed) Handbook of specialty elastomers. Taylor & Francis Group LLC, New York
Zheng J, Tan J, Gao H, Wang C, Dong Z (2014) Preparation of low temperature resistant and high electrical insulation chloroprene rubber-butadiene rubber blends. Rubber Chem Technol 87:360–369
Pilipovic A, Sercer M, Kodvanj J (2010) Influence of crosslinking parameters on mechanical properties of chloroprene rubber. Trans Famena 34:57–70
Smejda-Krzewicka A, Rzymski WM, Kowalski D (2015) Tin oxide cross-linking chloroprene rubber. Polimery 3:186–191
Brydson JA (1976) Chloroprene. In: Brydson JA (ed) Rubber chemistry. Applied Science Publishers, London
Rzymski WM, Bociong K, Pizon P, Kajkowski R (2014) Cross-linking of chloroprene rubber. Polish Patent PL 216,835, Poland
Janowska G, Rzymski WM, Smejda-Krzewicka A, Bociong K, Kowalski D (2013) The method of cross-linking and modifying of chloroprene rubber. Polish Patent PL 215,569, Poland
Smejda-Krzewicka A, Dmowska-Jasek P, Kobędza P (2016) The method of cross-linking of chloroprene rubber. Polish Patent Declaration P.415,996, Poland
Ismail H, Leong HC (2001) Curing characteristics and mechanical properties of natural rubber/chloroprene rubber and epoxidized natural rubber/chloroprene rubber blends. Polym Test 20:509–516
De SK, White JR (2001) Rubber technologist’s handbook. Rapra Technology, Shawbury
Szlezyngier W (1998) Plastics. Educational Publishing House FOSZE, Rzeszow
Maya MG, Soney CG, Thomasukutty J, Lekshmi K, Sabu T (2018) Development of a flexible and conductive elastomeric composite based on chloroprene rubber. Polym Test 65:256–263
Al-Maamori MH, Al-Mosawi A, Abbas Abdulsada S (2018) Effect of novolac nanoparticles additions on specific gravity of NBR/CR blends. In: MATEC web of conference, vol 178, p 04001
Mingyi L, Hua Z, Jianfeng I (1999) Study on the compatibility of BR/CR/SBR blends by using small amount of SBS. J Appl Polym Sci 71:215–220
Olejnik A, Smejda-Krzewicka A, Strzelec K, Szynkowska MI (2018) Curing and properties of chloroprene and butadiene rubber (CR/BR) blends cross-linked with copper(I) oxide or copper(II) oxide. Int J Polym Anal Charact 24:18–31
Smejda-Krzewicka A, Olejnik A, Dmowska-Jasek P, Strzelec K (2016) The method and modifying of chloroprene and butadiene rubber blends. Polish Patent Declaration P. 416,236, Poland
Posadas P, Malmierca MA, González-Jiménez A, Ibarra I, Rodríguez A, Valentin JL, Nagaoka T, Yajima H, Toki S, Che J, Rong L, Hsiao BS (2016) ESR investigation of NR and IR rubber vulcanized with different cross-linking agent. Express Polym Lett 1:2–14
Goyanes S, Lopez CC, Rubiolo GH, Quasso F, Marzocca AJ (2008) Thermal properties in cured natural/styrene butadiene rubber blends. Eur Polym J 44:1525–1534
Das A, Mahaling RN, Stöckelhuber KW, Heinrich G (2011) Reinforcement and migration of nanoclay in polychloroprene/ethylene-propylene-diene-monomer rubber blends. Compos Sci Technol 71:276–281
Jurkowski B, Jurkowska B, Rydarowski H (2009) Some aspects of flammability of polymer composition. Publishing House of Cracow University of Technology, Cracow
Hilado CJ (1998) Flammability handbook for plastics. Technomic Publishing Company, New York
Funding
Funding was provided by Politechnika Lódzka.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Smejda-Krzewicka, A., Olejnik, A. & Strzelec, K. The role of iron(III) oxide in chloroprene and butadiene rubber blends’ cross-linking, structure, thermal and mechanical characteristics. Iran Polym J 28, 313–323 (2019). https://doi.org/10.1007/s13726-019-00701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00701-x