Abstract
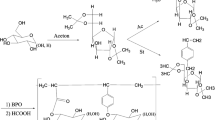
The present study describes the synthesis and characterizations of polymerizable vinyl sugars. Glucose, mannose, galactose and fructose are abundant and sustainable natural compounds. As it is not possible to make many derivatives of sugars without using protective groups, first of all, diacetone derivatives [diacetone-d-glucose (1), diacetone-d-mannose (2), diacetone-d-galactose (3) and diacetone-d-fructose (4)] were synthesized according to the literature as starting compounds. The remaining free hydroxyl groups on C-3 (diacetone glucose), C-6 (diacetone galactose), C-1 (diacetone fructose) and C-1 (diacetone mannose), were reacted with epichlorohydrin (1-chloro-2,3-epoxypropane) to produce then “-O-(2′,3′-epoxypropane-1′-yl)” ether derivatives (5, 6, 7, and 8) which are epoxy sugars in the basic medium. Next, the epoxy rings of the ethers (5, 6, 7, and 8) were opened with methacrylic acid in DMF to produce new sugar based methacrylates (9, 10, 11, and 12). Finally, free radical polymerization of these sugar based methacrylate monomers was performed, producing related polymers (13, 14, 15 and 16). The polymerizations were carried out using AIBN as an initiator at 70 °C in DMF. All the products were characterized by FTIR, 1HNMR and 13CNMR techniques. Thermal properties of all polymers were investigated by TG, DTG and DSC. The data obtained has suggested that thermal stability of the synthesized polymers has changed with the structure of the sugar and increase in molecular weight.






Similar content being viewed by others
References
Louwrier A (1998) Industrial products—the return to carbohydrate-based industries. Biotech Appl Biochem 27:1–8
Mazzolari GL, Cignoli A (1991) Fluidized-bed anaerobical reactor for the conditioning of agricultural and industrial effluents. ICP 19:47–54
Singh R, Bhattacharya B, Rhee HW, Singh PK (2015) Solid gellan gum polymer electrolyte for energy application. Int J Hydrogen Energ 40:9365–9372
Methven JM (1991) Polymeric materials from renewable resources. Rapra Rev Reports 4:1–134
Lin K, Kasko AM (2014) Carbohydrate-based polymers for immune modulation. ACS Macro Lett 3:652–657
Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine D (2010) Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16:1035–1041
Sunasee R, Adokoh CK, Darkwa J, Narain R (2014) Therapeutic potential of carbohydrate-based polymeric and nanoparticle systems. Expert Opin Drug Deliv 11:867–884
Garcia-Martin MG, Jiménez-Hidalgo C, Al-Kass SSJ, Caraballo I, de Paz MV, Galbis JA (2000) Synthesis and characterization of some new homo- and co-poly(vinylsaccharides). Some preliminary studies as drug delivery. Polymer 41:821–826
Bezouška K (2002) Design, functional evaluation and biomedical applications of carbohydrate dendrimers (glycodendrimers). Rev Mol Biotech 90:269–290
Knott BC, Crowley MF, Himmel ME, Ståhlberg J, Beckham GT (2014) Carbohydrate–protein interactions that drive processive polysaccharide translocation in enzymes revealed from a computational study of cellobiohydrolase processivity. J Am Chem Soc 136:8810–8819
Choi SK, Mammen M, Whitesides GM (1997) Generation and in situ evaluation of libraries of poly(acrylic acid) presenting sialosides as side chains as polyvalent inhibitors of influenza-mediated hemagglutination. J Am Chem Soc 119:4103–4111
Lees WJ, Spaltenstein A, Kingery-Wood JE, Whitesides GM (1994) Polyacrylamides bearing pendant.alpha.-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: multivalency and steric stabilization of particulate biological systems. J Med Chem 37:3419–3433
McReynolds KD, Gervay-Hague J (2007) Chemotherapeutic interventions targeting HIV interactions with host-associated carbohydrates. Chem Rev 107:1533–1552
Yoshida T, Akasaka T, Choi Y, Hattori K, Yu B, Mimura T, Kaneko Y, Nakashima H, Aragaki E, Premanathan M, Yamamoto N, Uryu T (1999) Synthesis of polymethacrylate derivatives having sulfated maltoheptaose side chains with anti-HIV activities. J Polym Sci A Pol Chem 37:789–800
Miura Y, Yasuda K, Yamamoto K, Koike M, Nishida Y, Kobayashi K (2007) Inhibition of alzheimer amyloid aggregation with sulfated glycopolymers. Biomacromolecules 8:2129–2134
Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schatzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtles S, Duncan R, Cassidy J (2009) Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancerInt. J Oncol 34:1629–1636
Black WAP, Dewar ET, Rutherford D (1963) Polymerisation of unsaturated derivatives of 1,2: 5,6-di-O-isoprop ylidene-d-glucofuranose. J Chem Soc 4433–4439
Imoto M, Kimura S (1962) Optically active polymers. II. Relation of the optical activity of polymethacryloyldiisopropylidene-d-glucose with its molecular weight. Makromol Chem 53:210–211
Kimura S, Hirai K (1962) Vinyl polymerization LIX. On the syntheses of polymethacrylates of d-glucose and l-sorbose. Makromol Chem 58:232–236
Saltan F, Akat H (2013) Synthesis and thermal degradation kinetics of d-(+)-galactose containing polymers. Polimeros 23:697–704
Rauter AP, Canda T, Justino J, Ismael MI, Figueiredo JA (2004) Synthesis of phenylseleno sugars from epoxides and of a, b-unsaturated carbonyl derivatives for the study of their insecticidal activity. J Carbohydr Chem 23:239–251
Stefan LM, Pana AM, Silion M, Balan M, Bandur G, Rusnac LM (2011) Efficient preparation and characterization of carbohydrate based monomers. d-mannose derivatives. Int Sch Sci Res Innov 5:284–288
Varma AJ, Kennedy JF, Galgalia P (2004) Synthetic polymers functionalized by carbohydrates: a review. Carbohyd Polym 56:429–445
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koruyucu, M., Saltan, F., Kök, G. et al. Synthesis, characterization and polymerization of novel sugars based on methacrylate. Iran Polym J 25, 455–463 (2016). https://doi.org/10.1007/s13726-016-0437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-016-0437-5