Abstract
Purpose of Review
This review examines lifestyle modification for obesity management with the goal of identifying treatment components that could support the use of a new generation of anti-obesity medications (AOMs).
Recent Findings
Semaglutide reliably reduces baseline body weight by approximately 15% at 68 weeks, in contrast to 5–10% for lifestyle modification. Tirzepatide induces mean losses as great as 20.9%. Both medications reduce energy intake by markedly enhancing satiation and decreasing hunger, and they appear to lessen the need for traditional cognitive and behavioral strategies (e.g., monitoring food intake) to achieve calorie restriction. Little, however, is known about whether patients who lose weight with these AOMs adopt healthy diet and activity patterns needed to optimize body composition, cardiometabolic health, and quality of life.
Summary
When used with the new AOMs, the focus of lifestyle modification is likely to change from inducing weight loss (through calorie restriction) to facilitating patients’ adoption of dietary and activity patterns that will promote optimal changes in body composition and overall health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A new generation of anti-obesity medications (AOMs), inaugurated in 2021 with the US Food and Drug Administration’s (FDA) approval of semaglutide 2.4 mg, holds real promise of transforming the management of obesity [1••]. These novel nutrient stimulated hormone-based therapies reduce baseline body weight by an average of 15% or more [2••, 3•]. The FDA recommends that semaglutide be used as an adjunct to a reduced calorie diet and increased physical activity [4], long considered the cornerstone of obesity management when combined with behavior-change strategies [5, 6]. The efficacy, however, of this medication and of the recently FDA-approved tirzepatide (November 8, 2023) raises questions about the specific diet and activity counseling needed. The intensity of traditional lifestyle modification likely can be reduced, but new diet and activity challenges may arise with the larger weight losses produced by what Garvey [7] has called “second-generation” AOMs. This review examines these and related issues after first summarizing the mechanisms of action and efficacy of both lifestyle modification and two new AOMs.
Lifestyle Modification for Overweight and Obesity
Lifestyle modification provides behavioral and cognitive strategies to help individuals consciously regulate their energy intake (i.e., food) and expenditure (i.e., physical activity) [8,9,10,11,12]. This approach fortifies the highly integrated gut-to-brain neuroendocrine system, which evolved in an environment of food scarcity (rather than abundance) to facilitate energy homeostasis and prevent the loss of body mass, vital to survival [13]. In the USA and other high-income nations, the neuroendocrine regulation of body weight has been largely overwhelmed for the past 40 years by what Brownell and Horgen have labeled a toxic food environment [14]. It relentlessly markets large portions of cheap, highly processed, and highly palatable foods (e.g., fat, sugar, salt) that excite neural reward pathways that encourage excess eating [14, 15]. This mismatch between our ancient appetite-regulatory system and the current food environment [16], combined with marked reductions in energy expenditure at work and at home [14], explains much of the obesity epidemic.
As described elsewhere, traditional lifestyle modification begins with daily monitoring of food intake and physical activity to educate individuals about energy balance [8,9,10,11,12]. Patients who are new to tracking are often surprised by the high calorie content of morning juices, fast-food lunches, and evening snacks and beverages, as well as by the modest calorie expenditure of a 30-min walk. Individuals are encouraged to reduce portion sizes, as well as excess fat and sugar, to decrease intake by 500–750 kcal/day [6]. This deficit typically can be achieved in persons who weigh < 250 lb by aiming for 1200–1499 kcal/day or 1500–1800 kcal/day if ≥ 250 lb [6, 10]. Consumption of a self-selected diet is recommended with approximately 15% of energy from protein, 20–35% from fat, and the remainder from carbohydrate, although this mix can be adjusted (e.g., 25% protein) to meet dietary preferences [6]. Strategies such as shopping from a list, storing foods out of sight, and preparing more meals at home (i.e., less ordering in and eating out) help distance individuals from the food environment [8,9,10,11,12]. Cognitive techniques facilitate satiation, coping with hunger and cravings, and recovery from dietary lapses. The initial activity goal is walking (or other aerobic activity) for ≥ 150 min/week [6, 17], eventually increasing to ≥ 250 min/week for weight-loss maintenance [18]. Participants are instructed to weigh themselves at least weekly and to use problem solving to adjust their eating and activity in response to their weight change [8,9,10,11,12].
End-of-Treatment Outcomes
Individuals must devote substantial time, thought, and planning to cognitive self-regulation [10]. This includes triumphing daily over exhortations (e.g., food advertising) to eat more, as well as increasing physical activity in an environment that implicitly discourages it. These efforts are supported by participating in a structured 1-year program that provides, in the first 6 months, at least 14 individual or group sessions with a trained interventionist [6, 8, 19]. (Treatment is often weekly for the first 3–6 months.) Such regimens typically induce a mean 5–10% reduction in baseline weight at 1 year (compared with 1–2% for controls), as in the Diabetes Prevention Program and Look AHEAD trials [6, 8, 20,21,22]. Approximately 55–65% of participants lose ≥ 5% of weight, 30–35% lose ≥ 10%, and 10–15% achieve ≥ 15% [23] (Fig. 1). These reductions are associated in a generally linear manner with improvements in cardiovascular disease (CVD) risk factors, quality of life, and prevention of type 2 diabetes [24,25,26]. Variation of macronutrient composition (e.g., carbohydrate, protein) has little effect on 1- to 2-year weight loss but may improve some health conditions (type 2 diabetes) [27, 28]. Combining lifestyle modification with high-protein, very-low-calorie diets (≤ 800 kcal/day) increases short-term weight loss to 12–20% but with more costs, side effects, and weight regain than less restrictive diets [29].
Post-Treatment Outcomes and Challenges
Limitations of lifestyle modification include the plateauing of weight loss at 6–9 months [30], even when participants still have obesity and may receive continued counseling [6, 31]. In addition, individuals regain a mean one-third of lost weight in the year following treatment discontinuation, with further regain over time [6, 30]. Monthly or more frequent weight-loss-maintenance therapy delays but does not prevent regain, particularly after treatment concludes [6, 30, 31]. Some individuals, as in the National Weight Control Registry, achieve and maintain losses ≥ 15%, but they work tenaciously [32]. Registry participants report eating 1381 kcal/day and engaging in 60 min/day of physical activity. The high activity level appears necessary to compensate for greater-than-expected reductions in energy expenditure, both at rest and during activity, which accompany weight loss (i.e., metabolic adaptation) [33,34,35]. A final shortcoming of lifestyle modification is its limited availability outside of academic medical centers (and some commercial and community-based programs), making it virtually impossible for primary care providers to offer all patients with obesity “intensive, multicomponent behavioral interventions,” as recommended by the US Preventive Services Task Force [36]. Digitally delivered interventions have increased the reach of lifestyle modification but with reduced efficacy to date [8].
Second-Generation AOMs: Mechanisms of Action and Safety Findings
Semaglutide 2.4 mg produces roughly twice the weight loss, on average, as traditional lifestyle modification and also represented a major advancement over the four previously approved medications for chronic weight management—orlistat, naltrexone-bupropion extended release (ER), phentermine-topiramate ER, and liraglutide 3.0 mg [37,38,39]. The last two medications are the most effective of the four, inducing mean 1-year reductions of 8–11% of baseline weight when combined with approximately monthly lifestyle counseling [37,38,39]. AOMs are considered potentially appropriate for individuals with a BMI ≥ 30 kg/m2 or a BMI ≥ 27 kg/m2 with an obesity-related comorbidity (e.g., hypertension). (We note that in 2020, the US FDA and other regulatory bodies approved setmelanotide for chronic treatment in adults and children at least 6 years of age with rare monogenic or syndromic obesities [40].)
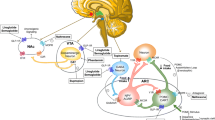
Liraglutide 3.0 mg and semaglutide 2.4 mg are both glucagon-like peptide 1 (GLP-1) receptor agonists [41]. GLP-1 is a native hormone that is released from L-cells of the small intestine and colon in response to nutrient (meal) intake. It binds to GLP-1 receptors expressed in pancreatic beta cells and the heart, as well as in appetite-regulating centers in the hindbrain, hypothalamus, and mesolimbic pathway [41, 42]. GLP-1 stimulates insulin secretion and inhibits glucagon release (in a glucose-dependent manner); it reduces energy intake by slowing gastric emptying and enhancing satiety signaling [41]. The half-life of native GLP-1 is approximately 2–3 min because of degradation by dipeptidyl peptidase 4 (DPP-4). Synthetic GLP-1 receptor agonists (GLP-1RA) are designed to resist this degradation. Liraglutide 3.0 mg has a half-life of approximately 13 h, thus, requiring daily subcutaneous dosing [41].
In contrast, semaglutide 2.4 mg has a half-life of about 180 h, which allows once-weekly subcutaneous dosing [1••, 3•]. This supraphysiologic dose of GLP-1 is associated with robust improvements in objective and subjective appetite control, in the absence of lifestyle counseling. At 20 weeks, semaglutide 2.4 mg, relative to placebo, reduced ad libitum energy intake by 35% during a laboratory lunch test meal. The mean reduction in energy intake, from baseline, was 377 kcal for semaglutide versus 152 kcal for placebo [42]. Semaglutide, relative to placebo, also resulted in larger self-reported reductions in hunger and food cravings, increased fullness and satiety, better control of eating, and lower preference for energy-dense foods [43].
Tirzepatide combines—in a single molecule—glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonism [2••]. At the time of this writing, the medication is approved in the US and European Union as a once-weekly subcutaneous injection for type 2 diabetes and, as noted, was recently approved in the US for overweight/obesity [3•]. The mechanisms by which tirzepatide’s dual receptor agonism increases weight loss, relative to GLP-1 agonism alone, are incompletely understood [44]. In adults with type 2 diabetes, tirzepatide 15 mg once weekly reduced energy intake, during an ad libitum lunch, from baseline to week 28, by 348 kcal compared to 39 kcal for placebo [45]. The reduction in energy intake with tirzepatide did not differ significantly from that in participants who received semaglutide 1 mg (with a reduction of 284 kcal). Tirzepatide was associated with significant improvements in self-reported appetite control compared with placebo [45]. By consistently reducing hunger (which initiates eating), increasing satiation and satiety (which terminate meal intake and extend the inter-meal interval, respectively), and potentially decreasing hedonic eating [45,46,47], the authors view the new-generation AOMs as reducing patients’ responsiveness—or vulnerability—to the toxic food environment.
We note that numerous other medications, with more complex mechanisms of action, are currently being developed [3•]. These include retatrutide, a triple-hormone-receptor agonist (GIP/GLP-1/Glucagon) [48], which was shown in a phase-2 study to reduce baseline weight by as much as 17.5% at 24 weeks and 24.2% at 48 weeks. Combining semaglutide 2.4 mg with an amylin-receptor agonist, cagrilintide 2.4 mg (i.e., CagriSema 2.4 mg), was similarly shown in a phase-1b trial to decrease baseline weight by 17.1% at 20 weeks, with substantial additional weight loss projected with continued use [49]. These and other medications are not discussed further due to space limitations and because their consideration for approval is more than a year away.
Safety
Across the phase 3 trials of semaglutide and tirzepatide (the latter still being completed), the most common adverse events were gastrointestinal in nature, including nausea, diarrhea, vomiting, constipation, dyspepsia, and abdominal pain [1••, 2••, 50, 51•, 52••, 53••, 54,55,56,57]. These symptoms were typically mild to moderate in severity. The rate of discontinuation due to adverse events was approximately 6–7% in participants treated by semaglutide 2.4 mg [1••] or by tirzepatide 10–15 mg [2••] vs 2.5–3% for placebo-treated participants. The rate of serious adverse events (SAEs) with semaglutide was approximately 7.7–9.8% vs 5.6–6.4% for placebo [1••], with similar rates for tirzepatide vs placebo [2••]. Adverse events of special interest included gallbladder-related disorders, acute pancreatitis, acute renal insufficiency, hypoglycemia, and injection site reactions [1••, 2••].
Semaglutide and tirzepatide are both titrated upward over 16 to 20 weeks to limit GI side effects described previously [1••, 2••]. Semaglutide, for example, is introduced at 0.25 mg for 4 weeks and then increased at 4-week intervals to 0.5, 1.0, 1.7, and 2.4 mg. In phase 3 trials, lower doses were allowed for maintenance if intolerable side effects occurred. In clinical practice, the dose may be increased even more slowly, according to patient preference, side effects, and progression of weight loss.
Efficacy of Semaglutide 2.4 mg for Overweight/Obesity
To date, the efficacy of semaglutide 2.4 mg has been evaluated in a series of seven trials called semaglutide treatment effect in people with obesity (STEP). (Results of an eighth trial have yet to be published.) Participants in all trials but STEP 3 (described later) received brief (15 min) lifestyle counseling visits approximately every 4 weeks to help them meet the study goals of achieving a 500-kcal/day deficit and ≥ 150 min/week of moderate to vigorous physical activity (principally walking). Counseling was delivered by registered dietitians (RD) or other qualified professionals who were free to advise participants as they saw fit. In some studies, the sponsor provided supplementary materials (e.g., food plate, toolbox) to be shared with participants at the RD’s discretion.
End-of-Treatment Outcomes in Selected Trials
In STEP 1, participants with overweight/obesity (but not type 2 diabetes) treated with semaglutide lost a mean 14.9% of baseline weight at 68 weeks, compared with 2.4% for placebo (both combined with 17 brief lifestyle visits) [1••]. Roughly 75% of semaglutide-treated participants lost ≥ 10% of weight, 50% lost ≥ 15%, and one-third reduced by ≥ 20% (Fig. 1). The approximately 15% loss in STEP 1 was replicated in five additional STEP trials that included similar participants but addressed other study questions [51•, 52••, 53••, 54, 55, 57] (Table 1). In STEP 2, participants with type 2 diabetes (and overweight/obesity) lost only 9.6% with semaglutide 2.4 mg [50]. Other AOMs have yielded comparably smaller weight losses in patients with versus without type 2 diabetes [58].
Weight loss with semaglutide was associated with clinically meaningful improvements in several cardiometabolic risk factors (e.g., blood pressure and triglyceride levels) and in self-reported physical function (Table 1). On November 11, 2023, Lincoff et al. reported that semaglutide 2.4 mg reduced the risk of major adverse cardiovascular events (MACE) by 20%, compared with placebo, in persons with overweight/obesity and a history of CVD (but not type 2 diabetes) [59]. This is the first such finding from a randomized controlled trial of an obesity treatment. The results are consistent with those for both liraglutide 1.8 mg and semaglutide 1.0 mg in persons with type 2 diabetes and CVD (or a high risk of it) [60]. The previously discussed Look AHEAD study did not reduce MACE in patients with type 2 diabetes and overweight/obesity, potentially because of insufficient weight loss [61].
Comparative Treatment Outcomes
Two STEP trials compared the efficacy of semaglutide with either another FDA-approved AOM or with intensive lifestyle modification. STEP 8 observed mean 68-week reductions in baseline weight of 15.8% with semaglutide versus 6.4% with liraglutide 3.0 mg (and 1.9% for pooled placebo) [55]. Semaglutide has not been compared directly with the older orlistat, naltrexone-bupropion, or phentermine-topiramate.
STEP 3 sought to maximize weight loss with semaglutide by combining it with intensive lifestyle modification [51•]. This approach was based on prior evidence of additive weight loss with combined behavioral and pharmacologic therapies [62]. All participants in STEP 3 received 30 brief visits with an RD over 68 weeks, which included, for the first 8 weeks, a 1000–1200-kcal/day meal-replacement diet, given the greater weight loss with this approach compared with an isocaloric, self-selected diet [11]. Participants who received this program, with placebo, lost approximately 8% of baseline weight at week 28, which declined to 5.7% at week 68, likely because of decreased counseling visits in later months. Participants assigned to semaglutide, with the same lifestyle intervention, lost 16.0% of baseline weight at trial completion and achieved significantly greater improvements than placebo-treated participants on multiple measures of cardiometabolic risk, as expected with greater weight loss (Table 1).
Extrapolating across STEP 1 and 3—with needed caution—the addition of intensive lifestyle modification and meal replacements (MRs) in STEP 3 appeared to increase early weight reduction with semaglutide but produced only marginally greater end-of-treatment weight loss (1.1 percentage points) than in STEP 1, in which semaglutide was delivered with less intensive counseling (17 visits and no MRs). The anticipated additive weight loss was not observed. Instead, the medication appeared to help individuals achieve the same long-term weight loss, regardless of the intensity of the initial lifestyle counseling. The two studies, taken together, also suggest that semaglutide (with approximately monthly lifestyle counseling) is approximately twice as effective as high-intensity lifestyle counseling alone (as provided in STEP 3), although a randomized controlled trial is needed to confirm this hypothesis and to compare the cost-effectiveness of the two approaches.
As discussed later, the optimal content and frequency of lifestyle counseling with semaglutide require further investigation. The medication’s efficacy in improving hunger, satiation, and other aspects of appetite control appears to decrease the short-term need for traditional behavioral strategies to achieve calorie restriction. The authors have heard multiple patients’ remark, “I’m just not thinking as much about food and eating,” a welcomed reduction in food preoccupation (“food noise”) and the usual cognitive demands of weight management. Semaglutide, a biological therapy, appears to modify eating behavior with greater ease and efficiency than traditional behavioral therapy. As a colleague recently noted, “These medications make lifestyle modification ‘a smaller ask’ for both patients and practitioners.”
Post-treatment Outcomes and Challenges
Individuals regain weight quickly after discontinuing semaglutide. A subset of 228 STEP-1 participants, who lost a mean 17.3% of baseline weight at week 68, regained an average of two-thirds of their loss (11.6 percentage points) 1 year after discontinuing medication [63••]. (The placebo group regained 1.9 percentage points of a 2.0% loss.) Previously improved cardiometabolic outcomes also reverted to baseline. These results mirror those from STEP 4 in which semaglutide-treated participants lost a mean 10.6% of baseline (run-in) weight in 20 weeks but, when randomly switched to placebo, regained 4.9% percentage points (about half) in the ensuing 48 weeks [52••]. In contrast, participants randomly assigned to remain on semaglutide lost an additional 6.8% of run-in weight. These two trials confirm the chronicity of obesity and the clear need for its long-term (indefinite) treatment, as with other chronic diseases [53••, 64]. STEP 5 illustrated the benefit of such care. Semaglutide-treated participants lost approximately 15% of baseline weight at 52 weeks and maintained this full loss at 104 weeks, while still receiving medication, and had expected improvements in cardiometabolic risk factors and quality of life [53••] (Table 1).
Despite its apparent superiority to lifestyle modification, semaglutide (as well as tirzepatide) shares challenges with the former approach, including that a majority of patients continue to have obesity after treatment. Participants’ mean BMI in STEP 5 declined from 38.6 to 32.8 kg/m2, understandably hastening the search for combination therapies to induce larger losses [3, 49]. A similar challenge concerns access to treatment, which is currently imperiled by semaglutide’s variable insurance coverage, for obesity, and by very high out-of-pocket costs in many countries (about $1300/month in the USA). These issues must be resolved to make chronic obesity management a reality. Once addressed, patients—and their practitioners—must embrace long-term adherence to second-generation AOMs, particularly in view of data showing discontinuation of earlier, less expensive medications after only 2 to 4 months [65].
Efficacy of Tirzepatide for Overweight and Obesity
The safety and efficacy of once-weekly tirzepatide for overweight and obesity are being evaluated in the SURMOUNT trials, with the results of three studies published at the time of this writing [2••, 56, 66].
End-of-Treatment Outcomes in Selected Trials
In the 72-week SURMOUNT-1 study, participants with overweight/obesity (but not diabetes) lost a mean 15.0% of baseline weight with tirzepatide 5 mg, 19.5% with 10 mg, and 20.9% with 15 mg, compared to 3.1% for placebo [2••]. (All participants received lifestyle counseling which included prescription of a 500-kcal/day deficit and ≥ 150 min/week of physical activity.) More than 75% of participants on the 10 and 15 mg doses lost ≥ 10% of baseline weight, more than 65% lost ≥ 15%, and fully 50% lost ≥ 20% (Fig. 1). In addition, 32.3% and 36.2% of these participants, respectively, lost ≥ 25% of baseline weight (not shown in Fig. 1). Tirzepatide-treated participants achieved significantly greater improvements in cardiometabolic risk factors and physical function than those on placebo.
In SURMOUNT-2, after 72 weeks of intervention, participants with type 2 diabetes and overweight/obesity lost 12.8% of baseline weight on tirzepatide 10 mg and 14.7% on tirzepatide 15 mg, compared to 3.2% for placebo [56]. These participants lost approximately five percentage points less body weight than those in SURMOUNT-1, who were free of type 2 diabetes. This finding parallels that for semaglutide, as discussed earlier. Data from these two SURMOUNT trials suggest that tirzepatide 10–15 mg produces an approximately five percentage point greater reduction in baseline weight than semaglutide 2.4 mg, a hypothesis that is currently being tested in a randomized controlled trial.
SURMOUNT-3 examined the effects of administering tirzepatide 10–15 mg/day after participants were first required to lose at least 5% of their initial body weight during a 12-week lead-in program that provided intensive lifestyle intervention [66]. The intervention included eight visits with an RD, who prescribed a 1200–1500-kcal/day diet, with up to two meal replacements/day, as well as 150 min/week of physical activity. The 579 participants who met the ≥ 5% weight loss criterion, and were otherwise eligible to continue in the study, lost a mean 6.9% of baseline weight during the 12-week program and were randomly assigned to tirzepatide (maximally tolerated dose of 10–15 mg) or placebo for 72 weeks. Tirzepatide-treated participants lost an additional 18.4% of weight from randomization to week 72, compared with a gain of 2.5% for those assigned placebo. (All participants received only quarterly RD visits during the randomized trial.) Approximately 65% of participants on tirzepatide, compared with 4.2% on placebo, lost 15% or more of their randomization weight. The additional weight reduction with tirzepatide was associated with further clinically meaningful improvement in several health outcomes (e.g., blood pressure, waist circumference, lipids, HbA1c, and physical function), which were beyond those achieved in the lead-in period and which were in sharp contrast to the deterioration in these measures in the placebo group. Tirzepatide also facilitated the maintenance of the original lead-in weight loss (of 6.9%): 94.0% of tirzepatide-treated participants compared to 43.8% of placebo maintained 80% or more of their initial 12-week loss. As measured from the start of the lead-in program to the end of the randomized trial—a total of 84 weeks—participants assigned to tirzepatide achieved a cumulative 24.3% reduction in baseline body weight, compared with 4.5% for those on placebo.
These findings suggest a useful treatment option for patients who, following successful weight loss with intensive lifestyle modification (e.g., 7–10%), need to reduce further to achieve optimal control of an obesity-related complication, such as obstructive sleep apnea. The new AOMs also could benefit “do-it-yourself losers” whose weight reduction plateaus after a 5% loss, despite their continued efforts and desire to lose more. Tirzepatide and semaglutide could be particularly beneficial with patients who are not responsive to intensive lifestyle modification, defined as losing < 2% of baseline weight after five weekly sessions with a trained interventionist [67]. Eligibility to participate in phase 3 trials of both medications required a “history of at least one self-reported unsuccessful dietary effort to lose weight.” This criterion, however, could be interpreted as a failure to lose weight and keep it off, versus not being able to lose weight initially. Controlled trials are needed to assess the benefits of second-generation AOMs in patients who are determined prospectively not to respond to an intensive lifestyle intervention.
Findings from SURMOUNT-3 also suggest that intensive lifestyle intervention and AOMs have additive weight-loss benefit when used sequentially, rather than concurrently, as they were in STEP 3. The additional 18.4% reduction in randomization weight, achieved with tirzepatide after the lead-in period, was only marginally smaller than the mean losses of 19.5% and 20.9% achieved in SURMOUNT-1 with tirzepatide 10 and 15 mg/day, respectively. Sequential, additive weight loss was similarly observed when participants who had lost 6.0% of baseline weight in a low-calorie diet run-in program were randomly assigned to liraglutide 3.0 mg or placebo. Additional weight loss with liraglutide, however, was only one-third of that observed with tirzepatide [68]. The effect of reversing the treatment sequencing, by introducing AOMs first, followed by intensive lifestyle intervention, has not been assessed to date, as discussed later.
Additional SURMOUNT trials are evaluating tirzepatide for inducing additional weight loss with medication continuation vs discontinuation after initial weight reduction with tirzepatide (SURMOUNT-4) and reducing MACE in patients with established CVD and overweight/obesity (SURMOUNT-MMO). Other studies are examining obstructive sleep apnea (SURMOUNT-OSA) and heart failure with preserved ejection fraction (SUMMIT).
Post-treatment Outcomes and Challenges
We anticipate that post-treatment results and challenges with tirzepatide will be generally similar to those discussed with semaglutide. Further evaluation will follow when the full set of SURMOUNT trials has been completed.
Knowledge Gaps with Semaglutide and Tirzepatide
The exemplary phase 3 trials of semaglutide and tirzepatide clearly reveal the robust weight losses and improvements in cardiometabolic risk factors produced by both medications. However, important questions remain concerning changes in dietary intake, physical activity, psychosocial function, and related outcomes that may accompany weight reduction with these medications. Answers to these questions should facilitate the development of specific guidelines for lifestyle counseling with the new AOMs. We believe that further study and guidance are perhaps most needed concerning changes in body composition.
Body Composition
Weight loss with semaglutide and tirzepatide is accompanied by favorable reductions in body fat [1••, 2••]. However, it is also accompanied by reduced lean body mass [1••, 2••, 45, 69, 70], which may influence factors that contribute to body weight regulation and other health outcomes. Lean body mass (LBM) is considered a significant driver of metabolic rate, with a reduction in lean tissue partially contributing to reduced daily energy expenditure [34]. The reduction in LBM may also influence the tonic drive to eat, which may further contribute to influences on body weight regulation [71]. Reduced LBM may be of additional clinical importance given its association with decreased bone mineral density and increased risk of fractures, as well as its relation to metabolic function (e.g., insulin sensitivity) and aerobic capacity [72, 73]. Adults 65 years and older are at increased risk of sarcopenia, characterized by an age-related decrease in skeletal muscle mass, with accompanying losses of strength and physical function [74, 75].
With intensive lifestyle interventions, approximately 15 to 25% of total weight loss is derived from LBM [76, 77]; this amount typically increases with greater energy restriction, as with very-low-calorie diets [77]. With the larger weight losses produced by Roux-en-Y gastric bypass, a systematic review found that the median reduction of lean mass was 31% of total weight [77]. Analyses of subsets of semaglutide- and tirzepatide-treated participants who completed assessments by dual-energy X-ray absorptiometry (DXA) did not include estimates of the percentage of weight loss from LBM. However, supplementary data published with STEP 1 reported that participants lost 10.40 kg of fat mass and 6.92 kg of LBM, suggesting that roughly 40% of total weight loss was derived from lean mass [1••]. This is a high value, which requires verification by a thorough statistical analysis and by checking the assessment methods used, given that placebo-treated participants lost 1.17 kg of fat and an unexpected 1.48 kg of LBM (i.e., roughly 56% loss from lean mass). Data from SURMOUNT-1, presented by Kushner et al. at the 2022 meeting of The Obesity Society, suggested that approximately 25% of weight loss with tirzepatide was derived from LBM and that there were no significant differences in this outcome according to age category [78]. In adults < 50, 50 to < 65, and ≥ 65 years of age, the loss of lean mass was estimated to comprise 27%, 25%, and 24% of total weight loss, respectively. These estimated values also require additional statistical analysis, and the findings should be interpreted with caution given the small number of older participants.
We acknowledge that interpretation of changes in body composition is challenging, particularly since weight gain and obesity are associated with increases in both fat mass and LBM; thus, some loss of LBM is to be expected with weight reduction [79]. In addition, reduced lean mass with weight loss could result primarily from a reduction in low-density/low-quality muscle compared to normal density muscle [80], a finding which could allay concerns. It also may be possible to combine semaglutide and tirzepatide with novel drugs such as bimagrumab, which appears to increase lean mass while reducing fat mass [81]. Large-scale, in-depth investigations of changes in body composition with second-generation AOMs should be a top priority, given the range and vast numbers of patients who are likely to be treated with these drugs in the coming years. Studies should seek to identify risk factors for the excessive loss of lean mass, including age and gender, as well as the rapidity and total amount of body weight lost.
Dietary Intake with Second-Generation AOMs
The large weight losses achieved in the STEP and SURMOUNT trials suggest that participants were largely successful in achieving the 500-kcal/day energy restriction prescribed. Data from the ad libitum buffet lunch meals, described earlier, provide further evidence of the medications’ efficacy in reducing energy intake [43, 45]. Yet, no results have been published to date concerning participants’ baseline dietary intake, including macronutrient distribution, and how it may have changed with weight loss facilitated by the medications. Similarly, although participants in some trials were instructed to monitor their food intake, findings have not been reported from these data concerning potential changes in the number of daily meals and snacks consumed, the timing of meals, or potential changes in problem eating [82]. We similarly do not know whether greater record keeping was associated with greater weight loss, as found in a trial of liraglutide [83]. We hope that such data will be published over time or, if not available, that additional studies will be conducted to reveal how patients change their food intake to lose weight. What, for example, is the relative importance for weight loss—and for improved cardiometabolic health—of participants’ decreasing their customary portion sizes, while potentially eating the same diet of energy-dense, highly processed foods, versus shifting their intake towards servings of lean protein, whole grains, and more fruits and vegetables? Do semaglutide and tirzepatide facilitate patients not only reducing their energy intake but also adopting a health-promoting dietary pattern that will improve specific cardiometabolic risk factors (e.g., elevated lipids levels) beyond the improvements conferred by weight loss alone? Studies that address these questions will also provide a better understanding of the optimal frequency and focus of lifestyle counseling required with the new AOMs. The recommendations that follow here—and in the next sections—are based on existing guidelines and our observations from clinical practice but await refinement with further research.
Diet Quality
Since second-generation AOMs substantially decrease the quantity of food consumed, it is important to counsel patients to increase the quality of the foods they eat. Brief dietary assessment can be conducted in clinical settings using 24-h recalls, dietary screeners such as the Rapid Eating and Activity Assessment for Participants (short version) [84], or food frequency questionnaires (Table 2). Patients can also monitor their dietary intake using a paper or electronic diary (e.g., MyFitnessPal) and review a few days’ records with their health care professional or an RD.
Studies are needed to evaluate the benefits of consuming specific types of foods with second-generation AOMs. The literature, however, on large, rapid weight losses with very-low-calorie diets and bariatric surgery has shown the importance of prioritizing servings of lean protein to help preserve LBM [85]. Thus, to mitigate the potential loss of LBM with AOMs, we provisionally recommend that all patients consume a minimum of 60 g/day of high-quality protein [85], with a target of 0.8 g/day of protein per kilogram of body weight [86]. Higher amounts of protein may be appropriate with patients with higher BMIs who resemble individuals who undergo bariatric surgery [85]. Daily inclusion of a high-protein shake(s) may help meet these targets. We further recommend a diet that promotes cardiometabolic health, with an emphasis on increased fruits, vegetables, fiber, and other nutrient-rich foods, combined with decreased consumption of foods that are high in saturated fat and/or sugar [87]. Dietary strategies can be based on the patient’s comorbidities, as well as sociocultural preferences [88], and incorporate reduced-calorie versions of the US Dietary Guidelines [87], a Mediterranean-style diet [89], the DASH diet [90], or a variety of other approaches [6, 88]. MyPlate, developed by the US Dietary Guidelines (available at MyPlate.gov), provides patients a simple, accessible strategy for consuming a healthy dietary pattern [87].
Nutrient deficiencies are a common concern with marked energy restriction, as with very-low-calorie diets and bariatric surgery and could occur with some patients who achieve substantial weight loss with AOMs [91, 92]. Micronutrients of specific concern include vitamin B12, folate, thiamin, magnesium, potassium, calcium, the fat-soluble vitamins (A, D, E, K), iron, copper, zinc, and selenium [91, 92]. In the absence of official guidelines for micronutrient supplementation with energy-restricted diets [91], practitioners may wish to recommend a daily multivitamin for all patients taking AOMs and provide appropriate assessment and referral for nutritionally at-risk patients [92].
Dietary Control of AOM-Related Side Effect
Dietary counseling with AOMs can also provide strategies to help mitigate common adverse effects of the medications, particularly nausea, diarrhea, vomiting, and constipation, which tend to be most prominent during dose escalation. GLP-1 agents do not interact with foods, but we suggest avoiding fatty, fried, greasy, and high-sugar foods for health reasons and to decrease GI side effects. Additional suggestions to decrease GI symptoms include consuming food slowly, having smaller meals, eating food that is light (and less spicy/acidic), avoiding eating too late at night, and maintaining hydration, with recommended fluid intakes of 2.2 L/day for women and 3 L/day for men. Patients should be informed of the signs and symptoms of dehydration (e.g., decreased urine, dizziness, dry mouth) and advised to keep non-caloric fluids with them and to drink slowly and frequently throughout the day if they experience nausea. Decreasing alcohol and caffeine can also help to avoid dehydration. This counseling, similar to that provided post-bariatric surgery patients, may help increase the tolerability of the medications and prevent treatment discontinuation.
Physical Activity with Second-Generation AOMs
The STEP and SURMOUNT trials both found that weight loss was associated with participants’ reports of improved physical function [1••, 2••]. This is positive news, but it is not known whether this improvement was associated with participants adopting a more physically active lifestyle. As with dietary-intake findings, few data have been published from these trials on potential changes in participants’ physical activity or cardiorespiratory fitness occurring with weight loss, an absence also detected by a recent meta-analysis of the literature [93]. This underscores the need to quantify these outcomes and to examine the health benefits of coupling physical activity and improved cardiorespiratory fitness with weight loss achieved with AOMs. Patients and practitioners should be mindful that physical activity, independent of weight status, is associated with reduced risks of mortality, numerous cardiometabolic conditions, some forms of cancer, functional disabilities, and other complications [17]. Successful weight loss with AOMs may help patients enjoy physical activity for its recreational, social, and health benefits alone, without the need to use exercise to achieve negative energy balance, as it is with lifestyle modification. One patient remarked that he no longer experienced “the dread of having to exercise in order to lose weight” but instead enjoyed the greater physical activity that AOM-induced weight loss had afforded him.
Increasing Physical Activity
We recommend that patients treated with second-generation AOMs seek to achieve a level of physical activity consistent with public health guidelines, with personalization of the activity plan based on the patient’s medical status and personal preferences [17]. These guidelines include the following:
-
Progression to the equivalent of at least 5 day/week of 30 min of moderate-intensity aerobic physical activity (e.g., brisk walking). This progression can occur over a period of 3 to 6 months, the activity can be divided over multiple shorter episodes each day rather than need to complete all 30 min in one continuous period, and it can include activities other than walking that may be more enjoyable or more appropriate based on the patient’s physical capacity. This level of activity should be sustained to assist with weight loss maintenance and may need to increase to ≥ 60 min per day, particularly if patients decrease their AOM use. Patients can use a variety of devices (e.g., smart phones, watches, step counters) to track their activity.
-
At least 2 days of muscle strengthening activities. This activity could be beneficial given the observed loss of lean mass with AOMs and may contribute to muscle strength that facilitates engagement in activities of daily living and a more physically active lifestyle. Muscle strengthening may be particularly important for older adults to reduce the risk of sarcopenia that contributes to reduced physical function.
-
Balance training. This activity is also recommended for older adults and may be useful for younger individuals during weight loss to enhance their kinesthetic awareness and contribute to safe mobility and physical activity.
Given the significant energy deficit induced by the new AOMs, patients should be advised to gradually increase their physical activity, to not exercise to exhaustion, and to maintain adequate hydration. Practitioners also should counsel patients on the appropriate progression of physical activity based on the presence of comorbidities or other concerns.
Effects of Strength Training
We agree with public health guidelines that recommend the inclusion of strength (resistance) training as part of physical activity [17]. However, there is currently limited evidence to demonstrate that adding such exercise to an AOM will prevent or minimize the reduction in LBM. For example, in response to a very-low-calorie diet (VLCD), which induced the loss of ~ 20% of baseline weight (~ 20 kg), the addition of structured exercise (i.e., randomization to aerobic, resistance, or aerobic plus resistance groups) did not preserve lean mass significantly better than the VLCD alone [94]. The same lack of benefit was observed in a similarly designed randomized study that produced a loss of ~ 15% with a 925-kcal/day meal-replacement diet [95]. More encouraging results were obtained in a VLCD study which induced a 16-kg weight loss. Tissue biopsy revealed muscle hypertrophy in participants who engaged in resistance training, compared with non-exercising controls, despite comparable weight losses and changes in body composition in the two groups [96]. In an observational analysis of Roux-en-Y gastric bypass patients (from a randomized trial), the greatest increase in walking was associated with the best preservation of skeletal muscle, measured at the thigh, and with less loss of total body mean mass [97]. When added to weight loss resulting from Roux-en-Y gastric bypass, walking also enhanced muscle quality [97]. These findings warrant further examination with weight loss induced by the new AOMs.
Future research can be guided, in part, by a study that examined the efficacy, for maintaining a 12% weight loss (achieved with a low-calorie diet run-in), of placebo, liraglutide 3.0 mg, exercise alone, or the combination of liraglutide plus exercise. At 1 year, the combination therapy was associated with significantly greater improvements than all other groups in body-fat percentage, cardiorespiratory fitness, and general heath perceptions [98]. These data provide eloquent testimony of the benefits of patients engaging in physical activity to improve their physical health and quality of life, not just their weight.
Behavioral and Psychosocial Issues, Including Terminating AOMs
Many questions remain about the frequency and content of the behavioral (i.e., lifestyle) counseling required with the new AOMs. Based on current evidence, we recommend that brief diet and physical activity counseling be provided on the approximately monthly schedule used in the STEP trials, with two visits during the first month. More frequent meetings do not appear necessary to induce a 15–20% weight loss with the new AOMs, and some visits likely can be conducted by telephone or video-conferencing without loss of efficacy [31, 99]. Asynchronous, digitally delivered programs, which involve minimal person-to-person contact, also are likely to be explored [100]. In traditional office practice, RDs initially would appear to be best prepared to provide lifestyle counseling, but a variety of primary care professionals including physicians, nurse practitioners, nurses, psychologists, and medical assistants have provided such care following a structured behavioral protocol [12]. If not on staff, we encourage primary care practices to establish consulting relationships with RDs, certified clinical exercise physiologists, and psychologists for patients who need additional support in these respective areas.
Selecting Weight Loss Goals and Related Outcomes
We begin all weight management efforts by first examining patients’ desire for treatment, their understanding of the nature and course of therapy they will receive, and the benefits, risks, and challenges that they can expect. This includes discussing how much weight they wish to lose and how they chose their particular number. Twenty years ago, lifestyle-treated participants typically reported desiring to lose 25% or more of their baseline weight [101], which was often followed by practitioners’ extolling the health benefits of a 5–10% reduction, the mean loss they could deliver at the time [8]. Losses of 15–25% now appear to be within reach with second-generation AOMs and will be particularly helpful in treating obesity-related complications such as obstructive sleep apnea, osteoarthritis, and non-alcoholic steatohepatitis (NASH), which require large reductions for optimal control [102]. In addition to enhancing their physical health, we ask patients to discuss other outcomes they hope to achieve with weight loss, including improvements in mobility, energy, and self-esteem, as well as engaging in new activities, such as learning to dance, play a new sport, or travel with family [12]. We encourage them to focus on their achievement—and enjoyment—of these functional goals rather than on reaching a number on the scale.
Obesity experts and health care professionals have much to learn about prescribing the new AOMs in clinical practice. The following hypothetical case illustrates the need for prudent clinical guidelines—beyond criteria provided by the FDA—which account for an individual’s age, sex, current health status (and history), medication use, and related factors. A 72-year old male with a BMI of 27.3 kg/m2 (weight of 190 lb, and height of 70 in) has elevated triglyceride levels. He has asked his doctor to prescribe semaglutide so that he can get down to his freshman-year weight (162 lb) for his 50th college reunion. He appears eligible for the medication but is it in the best interests of his health, given his age and risk of sarcopenia? With individuals such as these, practitioners may still wish to extoll the virtues of a more moderate 5–10% weight loss, even though larger reductions are possible. A larger weight loss may not always be a healthier weight loss [103]. In this case, the patient could achieve a therapeutic outcome with traditional lifestyle modification alone; an older, less robust AOM; a smaller dose of semaglutide (to limit weight loss); or with physical activity training alone to improve strength and conditioning. Such options may also be more appropriate for individuals who have obesity but are free of weight-related complications (i.e., metabolically healthy). Similarly, the benefits of more moderate weight loss, achieved at a low cost with traditional lifestyle modification, should not be overlooked in preventing type 2 diabetes [20] or in reducing mild elevations in blood pressure and other cardiometabolic risk factors [24].
Self-Monitoring of Diet and Physical Activity
Additional questions concern the recommended frequency of patients’ monitoring their food and energy intake, the cornerstone of traditional lifestyle modification (and a more challenging task than tracking physical activity). Participants in most of the STEP and SURMOUNT trials had relaxed requirements for dietary self-monitoring and still lost approximately 15 to 20% of baseline weight, to which the lifestyle counseling program appeared to contribute only 2.5–3.0 percentage points (as suggested by the results for placebo-treated participants). Thus, frequent dietary self-monitoring does not appear to be necessary for successful weight loss with second-generation AOMs, although better data are needed to reach firm conclusions.
The frequency, however, of self-monitoring—and of lifestyle intervention contacts—needed to adopt a healthier dietary pattern may be substantially greater than that required to lose weight. Individuals, for example, who eat a diet of principally fast foods—or other highly processed items—likely will lose weight on AOMs simply by reducing the quantity of such foods consumed (resulting from decreased hunger and increased satiety), with little self-monitoring. By contrast, greater education, planning, self-monitoring, and lifestyle counseling likely would be required to shift from a diet of fast foods to a Mediterranean-style pattern, discussed earlier, with its potentially greater health benefits (beyond weight loss). Moreover, some patients likely will need more than monthly lifestyle counseling visits to consistently achieve the daily recommended intake of protein, an outcome not reported in any of the phase 3 trials. Regular self-monitoring also facilitates increased physical activity [8,9,10,11,12]. Thus, we recommend frequent monitoring of food intake and physical activity, as well as AOM adherence—perhaps the most critical behavior—during the first month to help patients identify their daily success with treatment adherence and needed behavior change. Provision of at least two lifestyle counseling visits the first month should help facilitate achievement of these goals. The intensity of self-monitoring—and of lifestyle intervention contacts—can be adjusted thereafter based on the patient’s specific goals for diet and activity change.
Psychosocial Function
We believe that patients should be screened before treatment, as they were in phase 3 clinical trials, to determine that they are free of symptoms of current major depression and active suicidal ideation and behavior, as well as other significant psychopathology. These conditions can be assessed by interview, supported by screening instruments such as the Patient Health Questionnaire-9 (PHQ-9) [104] and the Columbia Suicide Severity Rating Scale [105]. In approving semaglutide, the FDA recommended that patients be monitored for the possible occurrence of these adverse events. This guidance was based on a history of such complications with some prior AOMs (e.g., rimonabant), although a warning was not provided for semaglutide per se [4]. We explain to patients why we ask about thoughts of possible self-harm, since some seem surprised, or even offended, by the questions.
The effects of semaglutide and tirzepatide on possible changes in mood and suicidal ideation (or behavior), as assessed in the STEP and SURMOUNT trials, have not been fully reported yet, as they were for liraglutide 3.0 mg, which had a generally favorable neuropsychiatric profile [106]. To date, no randomized controlled trials have reported consistently higher rates of any adverse psychiatric events in patients treated with semaglutide or tirzepatide compared with placebo [1••, 2••, 50, 51, 52••, 53••, 54,55,56]. However, at the time of this writing, the European Medicines Agency was examining post-marking surveillance data that included about 150 reports of possible cases of self-injury and suicidal thoughts in persons using GLP-1 receptor agonists (for type 2 diabetes or obesity) [107]. It is difficult with such data to determine whether the adverse events are potentially related to the medication’s use or are unrelated and would have occurred in its absence. Regardless of the possible causal relationship, immediate intervention is required with persons who report active suicidal ideation when it is characterized by a wish to die, an intent to act on the wish, and a specific plan to end one’s life. Practitioners unfamiliar with the assessment of suicide risk and how to respond to it can obtain further information by completing the on-lining training that accompanies the Columbia Suicide Severity Rating Scale (https://CSSRS.columbia.edu/training/training-options) [105].
We also screen for the presence of bulimia nervosa [108], which we believe is a contraindication to weight reduction and should prompt referral to an eating disorders specialist. We also check for a history of bulimia nervosa and anorexia nervosa, the latter which is rare in our patients seeking weight management. Further research, in collaboration with mental health professionals, is needed on the use of second-generation AOMs by patients with a history of either of these eating disorders to determine whether substantial weight loss triggers a recurrence of negative weight-related cognitions and behaviors and potentially of the full eating disorder. We would delay using semaglutide and tirzepatide with such individuals until appropriate guidance is available. Studies also are needed on the effects of second-generation AOMs on binge-eating disorder (BED), as well as on night eating syndrome, in persons with overweight and obesity. Participants with BED were excluded in some of the phase 3 studies discussed previously. However, this disorder was recently shown to improve in patients with overweight/obesity who were treated with naltrexone bupropion ER [109].
Psychosocial outcomes including quality of life (QOL) and mood generally improve with behavioral weight reduction, with larger losses typically associated with greater benefits [110, 111]. The STEP 1, 2, and 4 trials found greater improvements in weight- and physical health–related QOL at week 68 in patients treated with semaglutide compared with placebo [1••, 50, 52••]. Detailed findings concerning mood and other psychological outcomes await further reporting from the phase-3 trials, as described previously.
We expect that AOMs will produce improvements, on average, in many psychosocial outcomes. Nonetheless, we believe practitioners must be alert to the possibility of untoward psychosocial effects. Some individuals who achieve large weight losses, as with bariatric surgery, report unanticipated interpersonal challenges such as receiving unwelcomed comments about their shape and weight, being treated differently than when they weighed more (and felt ignored or invisible), renegotiating interpersonal relationships that centered around food, or terminating unhappy marriages [112]. A minority of patients, particularly those with a history of physical and/or sexual abuse, may subliminally experience excess weight as a protective factor and feel traumatically anxious or vulnerable after weight loss [113]. Reports in bariatric surgery patients of an elevated risk of suicidality, generally 3 or more years post-surgery, are rare but may be relevant to a small number of individuals who achieve substantial weight loss with AOMs [110]. As second-generation AOMs are increasingly used by the general population and potentially by individuals who have more severe depression or anxiety (or significant personality disorders) than study participants had, rates of psychiatric events could increase, as will be assessed by post-marketing surveillance by FDA and other regulatory bodies. We believe this possibility owes principally to the elevated current and lifetime rates of depression and anxiety in persons with obesity, particularly those with a BMI > 40 kg/m2, who often experience the greatest ill effects of weight-related stigmatization [99, 114, 115].
In cases in which significant psychosocial distress or dysfunction does not resolve with empathic support and reassurance from a primary care provider—and in which immediate intervention is not required, as with active suicidal ideation—we recommend that patients be referred to a mental health professional for further evaluation. Such referral may include a small number of individuals who wish to lose more weight after already having achieved a relatively low BMI (e.g., 20–22 kg/m2). We have observed that such individuals often do not experience improvements in body image or self-esteem, propelling them to seek further weight loss as a solution (a behavior also seen in individuals with anorexia and bulimia). Cognitive-behavioral treatment for body image is likely to be more effective [116].
Long-Term Use of AOMs and Potential Discontinuation
Most patients likely will benefit from remaining on AOMs indefinitely to facilitate the maintenance of lost weight, as reviewed earlier [53••, 64]. We believe it is important to engage patients, as treatment progresses, in the discussion of long-term medication adherence, rather than stating at the outset, “you’ll need to take this medication for the rest of your life.” We often say “you can take the medication for as long as it’s helpful,” giving patients a sense of choice and control. Practitioners can clarify that medication will help patients lose weight in the first year and then, in ensuing years, support the equally important work of “keeping it off.” We also think it is important to acknowledge patients’ statements that “the medication’s not working as well—or any longer” when their weight loss plateaus or their subjective appetite control declines [47, 117]. Acknowledgement of disappointment or frustration can be joined with recognition that “the medication is still working to help you keep off 30 lb and control your diabetes.”
Several factors may lead patients to consider discontinuing AOMs including (1) concerns about their long-term safety, (2) patients’ beliefs that they should now be able to control their weight on their own (a potential form of self-stigmatization), and (3) the continued high costs of AOMs for weight-loss maintenance. Costs potentially could be reduced by less frequent dosing (e.g., every other week), as suggested with other medications [118] or by switching to an older, less expensive AOM (e.g., phentermine-topiramate, liraglutide), although this latter approach needs to be tested in a controlled trial to assess weight-loss maintenance following termination of semaglutide or tirzepatide. Some of our patients also have taken drug holidays for a month or more, with knowledge that they can return to the medication when needed, such as during the winter holidays. Research is urgently needed on cost-effective methods of facilitating weight-loss maintenance with AOMs.
Individuals who elect to discontinue AOMs should know that patients, on average, regain about two-thirds of lost weight in the ensuing year, a disheartening and daunting statistic [63••]. However, we have observed clinically that a small minority of patients maintain much of their weight loss after medication withdrawal. For 2 to 3 months before stopping a second-generation AOM, patients should be assisted in adopting a behavioral weight loss maintenance program, consistent with that practiced by participants in the National Weight Control Registry [32]. This could include enrolling in a structured behavioral program, as offered at academic medical centers or by some commercial providers. Better understanding of the effects of terminating AOMs on subjective and objective aspects of appetite and eating behavior could help patients address challenges in the initial weeks and months after drug discontinuation. Gradually reducing the medication dose, over 2–3 months—much as it was gradually introduced—could help ease discontinuation. The challenges to follow with long-term behavioral weight control are well known [30,31,32,33,34,35].
Conclusions
Much like the discovery of highly effective medical therapies for hypertension, type 2 diabetes, and lipid disorders that transformed the management of these diseases, we believe that a new generation of nutrient stimulated hormone-based therapies has the potential to do the same for the treatment of obesity and its associated complications. Investigators and practitioners have much to learn about second-generation AOMs, including further understanding the mechanisms by which they consistently lower the point of body weight regulation by 15% or more [64]. Both semaglutide and tirzepatide, for example, appear to induce weight loss principally by reducing energy intake, but do they also attenuate reductions in resting and non-resting energy expenditure (i.e., metabolic adaptation) that thwart weight loss with diet and physical activity alone? As discussed throughout this paper, much also remains to be determined about how best to encourage patients to adopt healthier dietary patterns and physical activity routines, needed for optimal health, when such behaviors initially may not appear to be as important for weight loss with the new AOMs.
We look forward to the development of clinical practice guidelines, as well as standards of care, to ensure the best possible use of the new AOMs. Such guidelines, as successfully developed for the practice of metabolic and bariatric surgery [85, 110], must stress the importance of treating persons with overweight and obesity with respect, dignity, and compassion. This includes recognizing, assessing, and treating the many physical and psychosocial complications that some patients with obesity experience and which are not ameliorated by weight reduction alone. Multidisciplinary care is essential for such individuals.
Bold, persistent effort also will be required at a societal level to ensure that persons from socioeconomically disadvantaged populations, who disproportionately bear the burden of obesity and its health complications, have access to second-generation AOMs. This will include reducing the high costs of these medications [119] and assuring their coverage by public and private insurers [120]. Finally, our ability to treat obesity more effectively, on an individual level, does not diminish the dire need to ameliorate the obesogenic environment that continues to fuel this pandemic on a population level in the USA and other nations [121].
Data Availability
No new original data were collected or produced in completing this article. The authors reviewed and synthesized selective literature (as referenced in the article) and then provided their recommendations for combining lifestyle modification with anti-obesity medications. We have no new data to share with other researchers.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. This pivotal randomized controlled trial of once-weekly semaglutide (2.4 mg) for overweight/obesity reported a mean 14.9% reduction in baseline weight at 68 weeks (compared with 2.4% for placebo) with generally favorable tolerability and safety findings.
•• Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16. This pivotal trial of once-weekly tirzepatide (15 mg) for overweight/obesity reported a mean 20.9% reduction in baseline weight at 72 weeks (compared with 3.1% for placebo) with generally favorable tolerability and safety findings.
• Jastreboff AM, Kushner RF. New frontiers in obesity treatment: GLP-1 and nascent nutrient stimulated hormone-based therapeutics. Annu Rev Med. 2023;74:125–39. A variety of new drug targets are being explored for overweight/obesity beyond glucagon like polypeptide-1 (GLP-1) receptor agonists (i.e., semaglutide 2.4 mg) and combined glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists (i.e., tirzepatide 15 mg).
Wegovy (package insert). Plainsboro, NJ: Novo Nordisk, USA;2021.
National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl.):51S-210S.
Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22(S2):S1–410.
Garvey WT. New horizons: a new paradigm for treating to target with second-generation obesity medications. J Clin Endocrinol Metab. 2022;107(4):e1339–47.
Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235–51.
The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP). Description of lifestyle intervention Diabetes Care. 2002;25(12):2165–71.
Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71.
Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14(5):737–52.
Wadden TA, Tsai AG, Tronieri JS. A protocol to deliver intensive behavioral therapy (IBT) for obesity in primary care settings: the MODEL-IBT program. Obesity (Silver Spring). 2019;27(10):1562–6.
Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267–96.
Brownell KD, Horgen KB. Food fight: the inside story of the food industry, America’s obesity crisis, and what we can do about it. New York: McGraw-Hill; 2004.
Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67-77.e3.
Guyenet SJ. The hungry brain: outsmarting the instincts that make us overeat. New York: Flatiron Books; 2017.
U. S. Department of Health and Human Services. Physical activity guidelines for Americans, 2nd edition: US Department of Health and Human Services; 2018. Available from: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed 29 June 2023.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71.
Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N); 2011. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&NCAId=253. Accessed 9 July 2023.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring). 2009;17(4):713–22.
Haywood C, Sumithran P. Treatment of obesity in older persons—a systematic review. Obes Rev. 2019;20(4):588–98.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(15):1492.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6.
Kolotkin RL, Fujioka K, Wolden ML, Brett JH, Bjorner JB. Improvements in health-related quality of life with liraglutide 3.0 mg compared with placebo in weight management. Clin Obes. 2016;6(4):233–42.
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7.
Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum LD, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–57.
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73.
Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring). 2006;14(8):1283–93.
Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34(4):841–59.
Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–54.
Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–46.
Rosenbaum M, Leibel RL. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring). 2016;24(8):1620–9.
Ravussin E, Smith SR, Ferrante AW. Physiology of energy expenditure in the weight-reduced state. Obesity (Silver Spring). 2021;29(Supp 1):S31–8.
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604.
U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US preventive services task force recommendation statement. JAMA. 2018;320(11):1163–71.
Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–62.
Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2016;6(3):237–48.
Yanovski SZ, Yanovski JA. Progress in pharmacotherapy for obesity. JAMA. 2021;326(2):129–30.
Pressley H, Cornelio CK, Adams EN. Setmelanotide: a novel targeted treatment for monogenic obesity. J Pharm Technol. 2022;38(6):368–73.
Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol. 2019;10:155.
Tan Q, Akindehin SE, Orsso CE, Waldner RC, DiMarchi RD, Müller TD, et al. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front Endocrinol. 2022;13: 838410.
Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754–62.
Boer GA, Hay DL, Tups A. Obesity pharmacotherapy: incretin action in the central nervous system. Trends Pharmacol Sci. 2023;44(1):50–63.
Heise T, DeVries JH, Urva S, Li J, Pratt EJ, Thomas MK, et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care. 2023;46(5):998–1004.
Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6): e133429.
Wharton S, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity (Silver Spring). 2023;31(3):703–15.
Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity - a phase 2 trial. N Engl J Med. 2023. Epub ahead of print.
Enebo LB, Berthelsen KK, Kanam M, Lund MT, Rubino D, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilinitide with semaglutide 2.4 mg for weight management: a randomized, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–48.
Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–84.
• Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–13. This randomized trial attempted, with limited success, to boost 68-week weight loss with semaglutide 2.4 mg by combining it with an initial 8-week, 1000-kcal/day meal-replacement diet and 30 sessions of intensive behavior therapy (departing from the 17 sessions used in most studies of semaglutide).
•• Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–25. Following all participants loss of approximately 10% of baseline weight with 20 initial weeks of semaglutide 2.4 mg, this 48-week trial revealed marked regain in participants randomly switched to placebo versus continued weight loss in those assigned to remain on medication.
•• Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–91. Participants randomized to receive semaglutide 2.4 mg lost approximately 15% of baseline weight at 52 weeks, which was fully maintained at 104 weeks with continued use of the medication, supporting the model of chronic care for the chronic disease of obesity.
Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206.
Rubino DM, Greenway FL, Khalid U, O’Neil PN, Rosenstock J, Sørrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–50.
Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023:S0140–6736(23)01200-X. Epub ahead of print.
Chao AM, Tronieri JS, Amaro A, Wadden TA. Semaglutide for the treatment of obesity. Trends Cardiovasc Med. 2023;33(3):159–66.
Hollander P, Gupta AK, Plodowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9.
Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S; SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023. Epub ahead of print.
Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson JJ, Kahn SE, et al. Semaglutide effects on cardiovascular outcomes in people with overweight of obesity: SELECT rationale and design. Am Heart J. 2020;229:61–9.
Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54.
Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20.
•• Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konaki K, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553–64. A subset of patients in the randomized STEP 1 trial, who originally had lost 17.3% at 68 weeks, were found to regain a mean 11.6 percentage points of their lost weight 52 weeks after discontinuing medication, underscoring the likely need for most patients to use medications long-term.
Stunkard AJ. Anorectic agents lower a body weight set point. Life Sci. 1982;30(24):2043–55.
Hemo B, Endevelt R, Porath A, Stampfer MJ, Shai I. Adherence to weight loss medications; post-marketing study from HMO pharmacy data of one million individuals. Diabetes Res Clin Pract. 2011;94(2):269–75.
Wadden TA, Chao AM, Machineni S, Kushner R, Ard J, Srivastava G, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023. Epub ahead of print.
Unick JL, Hogan PE, Neiberg RH, Cheskin LJ, Dutton GR, Evans-Hudnall G, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring). 2014;22(7):1608–16.
Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al; NN8022–1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–51.
Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJ. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul). 2019;34(3):247–62.
McCrimmon RJ, Catarig AM, Frias JP, Lausvig NL, le Roux CW, Thielke D, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63(3):473–85.
Blundell JE, Gibbons C, Beaulieu K, Casanova N, Duarte C, Finlayson G, et al. The drive to eat in homo sapiens: energy expenditure drives energy intake. Physiol Behav. 2020;219: 112846.
Johnson KC, Bray GA, Cheskin LJ, Clark JM, Egan CM, Foreyt JP, et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the Look AHEAD randomized clinical trial. J Bone Miner Res. 2017;32(11):2278–87.
Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev. 2017;97(4):1351–402.
Evans W. What is sarcopenia? J Gerontol. 1995;50(special issue):5–8.
Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):412–9.
Jakicic JM, Rogers RJ, Lang W, Gibbs BB, Yuan N, Fridman Y, et al. Impact of weight loss with diet or diet plus physical activity on cardiac magnetic resonance imaging and cardiovascular disease risk factors: heart health study randomized trial. Obesity (Silver Spring). 2022;30(5):1039–56.
Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007;31(5):743–50.
Kushner RF, Aronne LJ, Stefanski A, Ahmad N, Mao H, Bunck MC, et al. Tirzepatide-induced weight loss is associated with body composition improvements across age groups. Presented in oral session at Obesity Week 2022 (Nov 1–4).
Foster GD, Wadden TA, Mullen JL, Stunkard AJ, Wang J, Feurer ID, et al. Resting energy expenditure, body composition, and excess weight in the obese. Metabolism. 1988;37(5):467–72.
Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–47.
Heymsfield SB, Coleman LA, Miller R, Rooks DS, Laurent D, Petricoul O, et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open. 2021;4(1): e2033457.
Chao AM, Wadden TA, Walsh OA, Gruber KA, Alamuddin N, Berkowitz RI, et al. Effects of liraglutide and behavioral weight loss on food cravings, eating behaviors, and eating disorder psychopathology. Obesity (Silver Spring). 2019;27(12):2005–10.
Tronieri JS, Fabricatore AN, Wadden TA, Auerbach P, Endahl L, Sugimoto D, et al. Effects of dietary self-monitoring, physical activity, liraglutide 3.0 mg, and placebo on weight loss in the SCALE IBT trial. Obes Facts. 2020;13(6):572–583.
Segal-Isaacson CJ, Wylie-Rosett J, Gans KM. Validation of a short dietary assessment questionnaire: the Rapid Eating and Activity Assessment for Participants short version (REAP-S). Diabetes Educ. 2004;30(5):774, 776, 778 passim.
Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. 2005. Washington, DC: The National Academies Press. https://doi.org/10.17226/10490.
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at: DietaryGuidelines.gov.
Chao AM, Quigley KM, Wadden TA. Dietary interventions for obesity: clinical and mechanistic findings. J Clin Invest. 2021;131(1): e140065.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25): e34.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24.
Patel JJ, Mundi MS, Martindale RG, Hurt RT. Obesity. In: Mueller CM, editor. The ASPEN adult nutrition core curriculum. 3rd ed. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition; 2017. p. 1028–58.
Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health nutritional guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis. 2017;13(5):727–41.
Jobanputra R, Sargeant JA, Almaqhawi A, Ahmad E, Arsenyadis F, Webb DR, et al. The effects of weight-lowering pharmacotherapies on physical activity, function and fitness: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2023;24(4): e13553.
Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM. Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. Am J Clin Nutr. 1991;54(1):56–61.
Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65(2):269–77.
Donnelly JE, Sharp T, Houmard J, Carlson MG, Hill JO, Whatley JE, et al. Muscle hypertrophy with large-scale weight loss and resistance training. Am J Clin Nutr. 1993;58(4):561–5.
Carnero EA, Dubis GS, Hames KC, Jakicic JM, Houmard JA, Coen PM, et al. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity (Silver Spring). 2017;25(7):1206–16.
Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719–30.
Pearl RL, Wadden TA, Bach C, LaFata EM, Gautam S, Leonard S, et al. Long-term effects of an internalized weight stigma intervention: a randomized controlled trial. J Consult Clin Psychol. 2023;91(7):398–410.
Thomas JG, Panza E, Espel-Huynh HM, Goldstein CM, O’Leary K, Benedict N, et al. Pragmatic implementation of a fully automated online obesity treatment in primary care. Obesity (Silver Spring). 2022;30(8):1621–8.
Foster GD, Wadden TA, Phelan S, Sarwer DB, Sanderson RS. Obese patients’ perceptions of treatment outcomes and the factors that influence them. Arch Intern Med. 2001;161(17):2133–9.
Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE obesity clinical practice guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016 Jul;22 Suppl 3:1–203.
Wing RR, Neiberg RH, Bahnson JL, Clark JM, Espeland MA, Hill JO, et al. Weight change during the postintervention follow-up of Look AHEAD. Diabetes Care. 2022;45(6):1306–14.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77.
O’Neil PM, Oroda VR, Astrup A, Kushner R, Lau DCW, Wadden TA, et al. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529–36.
European Medicines Agency. EMA statement on ongoing review of GLP-1 receptor agonists; July 11, 2023. Available from: https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-receptor-agonists. Accessed 9 Oct 2023.
Yanovski SZ, Marcus MD, Wadden TA, Walsh BT. The Questionnaire on Eating and Weight Patterns-5: an updated screening instrument for binge eating disorder. Int J Eat Disord. 2015;48(3):259–61.
Grilo CM, Lydecker JA, Fineberg SK, Moreno JO, Ivezaj V, Gueorguieva R. Naltrexone-bupropion and behavior therapy, alone and combined, for binge-eating disorder: randomized double-blind placebo-controlled trial. Am J Psychiatry. 2022;179(12):927–37.
Sogg S, Lauretti J, West-Smith L. Recommendations for the presurgical psychosocial evaluation of bariatric surgery patients. Surg Obes Relat Dis. 2016;12(4):731–49.
Pearl RL, Wadden TA, Tronieri JS, Berkowitz RI, Chao AM, Alamuddin N, et al. Short-and long-term changes in health-related quality of life with weight loss: results from a randomized controlled trial. Obesity. 2018;26(6):985–91.
Sogg S, Gorman MJ. Interpersonal changes and challenges after weight-loss surgery. Prim Psychiatry. 2008;15(8):61–6.
Felitti VJ. Childhood sexual abuse, depression, and family dysfunction in adult obese patients: a case control study. Southern Med J. 1993;86(7):732–6.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx WJH, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–9.
Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the third national health nutrition examination survey. Am J Epidemiol. 2003;158(12):1139–47.
Schwartz MB, Brownell KD. Obesity and body image. Body Image. 2004;1:43–56.
Tronieri JS, Wadden TA, Walsh O, Berkowitz RI, Alamuddin N, Gruber K, et al. Effects of liraglutide on appetite, food preoccupation, and food liking: results of a randomized controlled trial. Int J Obes. 2020;44(2):353–61.
Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1(5588):352–4.
Levi j, Wang J, Venter F, Hill A. Estimated minimum prices and lowest available national prices for antiobesity medications: improving affordability and access to treatment. Obesity (Silver Spring). 2023;31(5):1270–79.
Baig K, Dusetzina SB, Kim DD, Leech AA. Medicare Part D coverage of antiobesity medications-challenges and uncertainty ahead. N Engl J Med. 2023;388(11):61–3.
Bray GA, Kim KK, Wilding JPH, World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–23.
Funding
JST’s effort on this article was supported by a mentored patient-oriented research award from the National Institutes of Health/National Institutes of Diabetes and Digestive and Kidney Disease (K23DK116935).
Author information
Authors and Affiliations
Contributions
TAW conceived of the review in consultation with all authors, who shared their expertise in dietary intervention (AMC, SL), physical activity and body composition (JMJ), behavioral and psychological interventions (JT, TAW), anti-obesity medications and patient care (AA, AMC, AG, TAW), and in searching the relevant literatures (all authors including MM). All authors helped to draft sections of the manuscript related to their area(s) of expertise, noted above, and MM developed the figure and tables. TAW edited the full manuscript, which was reviewed by all authors for critical revision. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Preparation of this selective review did not involve the collection of original research data from human participants. We reviewed and synthesized the existing literature (as identified in the article), derived our conclusions, and provided our recommendations for combining lifestyle modification with anti-obesity medications. In the absence of collecting original data, preparation of the present selective review was not examined by an institutional review board, consistent with the policies of the University of Pennsylvania and other academic institutions.
Human and Animal Rights and Informed Consent
This article cites several first authored papers by TAW, AMC, JST, and JMJ which describe the results of randomized clinical trials of obesity management interventions. In all cases, the studies were reviewed and approved by institutional review boards at the authors' home institutions (University of Pennsylvania for TAW, AMC and JST and University of Pittsburgh for JMJ). The studies were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interests
Thomas Wadden serves on scientific advisory boards for Novo Nordisk and WW (Weight Watchers) and has received grant support, on behalf of the University of Pennsylvania, from Eli Lilly, Epitomee Medical, and Novo Nordisk. Ariana Chao has served as a consultant to Eli Lilly and received grant support, on behalf of the University of Pennsylvania, from Eli Lilly and WW (Weight Watchers). Jena Tronieri has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. Anastassia Amaro has served on advisory boards for Fractyl Laboratories, Medality Medical, and Novo Nordisk and has received grant support, on behalf of the University of Pennsylvania, from Altimmune, Eli Lilly, and Fractyl Laboratories. Sharon Leonard has served as a consultant to Eli Lilly and Epitomee Medical. John Jakicic has received grant support, on behalf of Kansas University Medical Center, from Epitomee Medical. The other authors have no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wadden, T.A., Chao, A.M., Moore, M. et al. The Role of Lifestyle Modification with Second-Generation Anti-obesity Medications: Comparisons, Questions, and Clinical Opportunities. Curr Obes Rep 12, 453–473 (2023). https://doi.org/10.1007/s13679-023-00534-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00534-z