Abstract
Purpose of Review
To summarize the results of adult obesity neuroimaging studies (structural, resting-state, task-based, diffusion tensor imaging) published from 2010, with a focus on the treatment of sex as an important biological variable in the analysis, and identify gaps in sex difference research.
Recent Findings
Neuroimaging studies have shown obesity-related changes in brain structure, function, and connectivity. However, relevant factors such as sex are often not considered.
Summary
We conducted a systematic review and keyword co-occurrence analysis. Literature searches identified 6281 articles, of which 199 met inclusion criteria. Among these, only 26 (13%) considered sex as an important variable in the analysis, directly comparing the sexes (n = 10; 5%) or providing single-sex/disaggregated data (n = 16, 8%); the remaining studies controlled for sex (n = 120, 60%) or did not consider sex in the analysis (n = 53, 27%). Synthesizing sex-based results, obesity-related parameters (e.g., body mass index, waist circumference, obese status) may be generally associated with more robust morphological alterations in men and more robust structural connectivity alterations in women. Additionally, women with obesity generally expressed increased reactivity in affect-related regions, while men with obesity generally expressed increased reactivity in motor-related regions; this was especially true under a fed state. The keyword co-occurrence analysis indicated that sex difference research was especially lacking in intervention studies. Thus, although sex differences in the brain associated with obesity are known to exist, a large proportion of the literature informing the research and treatment strategies of today has not specifically examined sex effects, which is needed to optimize treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing concern worldwide and impacts the quality of life. In the USA, the prevalence of obesity among adults was 42.4% in 2017–2018 [1]. Obesity is associated with major health risks, such as high blood pressure, high cholesterol, type II diabetes, arthritis, asthma, and poor overall health [2]. However, there are differences in health risks and treatment outcomes between men and women with obesity [3]. For example, in a meta-analysis, men had a greater risk for mortality with increasing body mass index (BMI) compared to that for women (hazard ratio: 1.51 vs 1.30 per 5 kg/m2, p < 0.00001) [4]. Examining sex differences in obesity provides important information that can be used to inform treatment regimens tailored to each sex. However, more research is needed to understand the biological basis for the influence of sex in obesity [3].
Numerous neuroimaging studies have shown associations between obesity and measures of various brain features, such as morphology, resting-state activity and connectivity, white matter microstructure, and task activation. However, the effects may vary according to sex. Although research has confirmed the involvement of the brain in obesity, a comprehensive multimodal summary of the effects of both obesity and sex on the brain is lacking. Therefore, this review aimed to summarize the results of adult obesity neuroimaging studies published from 2010, with a focus on the treatment of sex in the analysis. Specifically, studies were categorized according to whether the analysis comprised direct comparisons between men and women (direct sex); used data from only male or only female participants or separately analyzed male and female participants, providing disaggregated data and allowing qualitative comparisons (single/within sex); controlled for sex, disallowing comparisons (sex adjusted); or did not consider sex, disallowing comparisons (no sex). We considered the first two categories to adequately treat sex as an important biological variable in the analyses. Additionally, we performed a keyword co-occurrence analysis of the selected articles, as a snapshot of popular topics/concepts in the field of adult obesity neuroimaging their relationship to the topic of sex differences.
Materials and Methods
Database Search Strategy and Results
We searched three online electronic databases, PubMed, Embase, and Web of Science, for obesity neuroimaging studies published from 2010 to the present. This search was conducted using a predetermined set of words related to obesity, neuroimaging, and sex/gender. The search terms, selected in collaboration with a research librarian, are indicated in Supplemental Table S1.
Eligibility Screening
Two stages of screening with predetermined inclusion and exclusion criteria were performed. The authors were contacted as needed. All studies considered for eligibility were published in a peer-reviewed journal.
In the first stage, we examined the title and abstract of each article for the following criteria: (1) investigation of obesity, (2) adult human participants, and (3) reporting of brain MRI data. Reasons for exclusion were as follows: (1) used non-human models; (2) included infant, child, or adolescent participants; (3) imaged non-brain tissues; (4) no structural magnetic resonance imaging (MRI), resting-state MRI (rsMRI), task/stimulus functional MRI (fMRI), or diffusion tensor imaging (DTI) was performed; (5) no measure of obesity was reported; (6) in treatment studies, no pre-treatment MRI data was reported; (7) investigated patients with anorexia nervosa or other eating disorders; (8) review article/protocol study/abstract only; and (9) duplicate article that was not previously identified.
In the second screening stage, we read the entire article, paying particular attention to the methods, results, and discussion sections. Articles that were found to violate the abovementioned study criteria upon full-text review were excluded.
Data Extraction
After screening, we extracted data on the treatment of sex in the analyses, participant characteristics (sex, population), modality(s) used, and main findings. Articles were categorized according to how the researchers analyzed sex. The first category was “direct sex,” which was applied to studies that directly compared men and women in the analysis. The second category was “single/within sex,” which was applied to studies that included only male or only female participants or separately reported results for male and female participants, without a direct comparison, but allowing qualitative comparisons. The third category was “sex adjusted,” which was applied to studies that controlled for sex differences in the analysis, disallowing comparisons between the sexes. The fourth category was “no sex,” which was applied to studies that did not consider sex in the analysis (at most, the numbers of male and female participants were reported), disallowing comparisons between the sexes. The first two categories were considered to adequately treat sex as an important biological variable in the analyses. Subsequently, we organized the findings by the type of brain imaging performed, namely, DTI, structural MRI, rsMRI, and task/stimulus fMRI, with multimodal studies represented in multiple sections.
Keyword Co-occurrence Analysis
A keyword co-occurrence network analysis of the included articles was conducted to examine popular topics/concepts in the field of adult obesity neuroimaging linked and not linked to the topic of sex differences. Keywords comprised those assigned to the article by the authors and/or the database (via various algorithms), with preference given to the keywords assigned by the Web of Science database for articles in multiple databases. Keyword co-occurrence networks represent each keyword as a node and each co-occurrence of a pair of words as a link, with the number of times that a pair of words co-occurs in multiple articles as the weight of the link connecting the pair. Keyword co-occurrence networks can be constructed to visualize the cumulative knowledge of a research area, aiding in uncovering meaningful knowledge and insights based on the patterns and strength of links between keywords that appear in the literature [5]. The citation records of the included articles were analyzed in VOSviewer (https://vosviewer.com) using LinLog normalization and modularity clustering techniques [6]. Other settings used in VOSviewer were as follows: counting method, fractional; attraction, 1; repulsion, 0; resolution, 1.1; minimum cluster size, 6. The top 100 keywords, based on the total strength of the co-occurrence links with other keywords (i.e., keywords frequently co-assigned to adult obesity neuroimaging articles), were selected.
Results
Study Selection
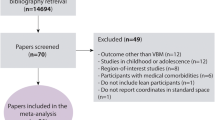
The PRISMA diagram illustrating the study selection process is shown in Fig. 1. In total, 190, 4982, and 1436 articles were retrieved from the PubMed, Embase, and Web of Science databases, respectively. These articles were imported into Endnote, and duplicates (n = 327) were removed. Thus, 6281 articles remained for further screening. After stage 1 screening, 5997 articles were excluded, and 284 articles were selected for full-text screening.
After second-stage screening, an additional 85 articles were excluded. The reasons for exclusion are indicated in Supplemental Table S2. The remaining 199 articles were categorized according to the treatment of sex in the analysis. Ten studies (5%) were categorized as direct sex, 16 studies (8%) as single/within sex, 120 studies (60%) as sex adjusted, and 53 studies (27%) as no sex (Fig. 2). The full bibliography according to article type is provided in the Supplemental Materials.
Pie chart showing the numbers and relative proportions of article types according to the treatment of sex in the analysis. Direct sex: the analysis comprised direct comparisons between men and women; single/within sex: the analysis used data from only male or only female participants or separately analyzed male and female participants; sex adjusted, the analysis controlled for sex; no sex: the analysis did not consider sex
Structural MRI
Direct-Sex and Single/Within-Sex Studies
In total, 12 studies reported on sex differences/commonalities and single/within-sex obesity-related alterations in regional and total gray matter volume (GMV), regional white matter volume (WMV), and cortical thickness. Although most of these studies reported associations between obesity (based on BMI cut-off points), or higher values in measures of body size (e.g., BMI, waist-to-hip ratio) or total body fat, and lower GMV in both sexes, the spatial extent of the alterations may be more widespread in men than in women [7–9], suggesting sex differences in the robustness or severity of morphological alterations. For example, one study found negative associations between total body fat and subcortical GMV in the thalamus, caudate nucleus, putamen, globus pallidus, hippocampus, and nucleus accumbens in men, while only the globus pallidus was affected in women [10]. Furthermore, an association between obesity and increased pituitary gland volume only reached statistical significance in men [11]. Similarly, relationships between obesity/larger waist-to-hip ratio and cortical thickness in frontal, temporal, and occipital cortices were found in men, while no such relationships were found in women [12, 13].
Among patients with cerebral small vessel disease, reductions in hippocampal volume over a 9-year period were more prominent in men than in women with larger baseline waist circumference [8]. In contrast, another study reported that reductions in hippocampal volumes were more severe in women than in men with type II diabetes (T2DM) and obesity [14]. Furthermore, a study on structural brain networks (based on cortical thickness and GMV) found that visceral adipose tissue volume exacerbated the effect of aging on network covariance in both men and women, which was modulated by the estradiol level in women but not men [15].
A female-specific study found a positive association between high BMI and GMV in the left nucleus accumbens [16], unlike the abovementioned study conducted in patients with T2DM [14]. Another female-specific study found associations between BMI and decreased GMV in the left orbitofrontal (associated with worse executive function), right inferior frontal, right precentral gyri, parahippocampal, fusiform, and lingual gyri, and right cerebellar regions, as well as increased WMV in the frontal, temporal, and parietal lobes in older women [17]. A male-specific study found a relationship between total brain volume and higher resting energy expenditure (protective against obesity) in Malaysian men, but not in Chinese and Asian-Indian men living in Singapore [18].
Sex-Adjusted Studies
Sex-covariate studies from 2010 to present (n = 48) reported on obesity-related alterations in morphological parameters, as well as changes before/after intervention. Similar to the direct-sex and single/within-sex studies, sex-adjusted studies generally support obesity/high-BMI/larger waist circumference as related to lower total GMV [19–26]; however, this may be an over-simplification, given the abovementioned findings regarding a sex difference in the spatial extent of GMV alterations. Specific regions with obesity-related (e.g., high BMI, abdominal obesity, obesity-prone status) GMV reduction include the thalamus and hippocampus [22, 27–30], which may have more robust obesity-related GMV reduction in men, based on the above-mentioned direct-sex and single/within-sex findings [10]. Additionally, cortical thickness alterations have been reported, with an overall thinner cortex and reduced regional cortical thickness in the ACC and posterior parietal cortices associated with obesity/higher BMI/larger waist circumference [31, 32]; these regions differ from those reported in direct-sex and single/within-sex studies [12, 13].
No-Sex Studies
No-sex structural studies from 2010 (n = 11) largely focused on the impact of bariatric surgery and other interventions on morphological parameters. These studies generally suggest that interventions can at least partially ameliorate obesity-related effects on these parameters [33–42].
rsMRI
Direct-Sex and Single/Within-Sex Studies
Direct-sex and single/within-sex rsMRI studies (n = 4) reported on regional alterations in functional centrality (a graph theory parameter) and the amplitude and frequency distribution of spontaneous fluctuations. A graph theory study reported that increased early life adversity (ELA) was associated with increased centrality in reward and emotion regulation regions in women with high BMI and in somatosensory regions in men with high BMI [43]. Another study found associations between BMI and altered spontaneous fluctuation frequency distribution in subcortical reward-related regions, including the left globus pallidus and substantia nigra, in both men and women, and additional alterations in the right globus pallidus and bilateral putamen in women; furthermore, BMI was associated with reduced connectivity between left globus pallidus/putamen and emotion and cortical regulation regions in women but not men [44].
Additionally, a female-specific study found that high BMI was associated with altered frequency distribution in the nucleus accumbens, ACC, and ventral PFC/orbitofrontal cortex [16]. Another female-specific study found an association between obesity and increased flow-frequency activity in the putamen, claustrum, and insula under fasting and fed states [45].
Sex-Adjusted Studies
Sex-adjusted rsMRI studies (n = 13) have mostly reported on alterations in intrinsic connectivity between regions or within a network, not centrality or frequency distribution changes, rendering it difficult to compare the results to those in direct-sex and single/within-sex studies. However, several studies reported obesity-related increases in intrinsic connectivity or involving regions with increased centrality or altered frequency distribution in direct-sex and single/within-sex studies, including the ventral striatum, amygdala, and insula [46–49].
No-Sex Studies
No-sex resting-state studies (n = 13) largely reported on the impact of interventions on rsMRI parameters [40, 41, 50–54].
fMRI Task/Stimulus
Direct-Sex and Single/Sithin-Sex Studies
Direct-sex and single/within-sex task-fMRI studies (n = 9) investigated obesity-related responses to high and low energy–dense foods, under fed and fasting conditions. In a fed state, men with obesity showed greater connectivity between the amygdala and subgenual ACC and between the ventral striatum and bilateral supplementary and primary motor areas, left postcentral gyrus, and left precuneus, and greater activation in bilateral supplementary motor areas (motor control), while women with obesity showed greater connectivity between the amygdala and the left angular gyrus and primary motor areas, and greater activation in the dorsal ACC, in response to high energy–dense food cues [55, 56]. In contrast, in a fasted state, men with obesity showed greater connectivity between the amygdala and bilateral supplementary frontal and primary motor areas, left precuneus, and right cuneus, and greater activation in a visual perception region (temporal-occipital cortex), while women with obesity showed greater connectivity between the amygdala and the inferior frontal gyrus and dorsomedial PFC, and greater/more robust activation in affective and reward-related regions (amygdala, insula, medial orbitofrontal cortex, etc.), in response to high energy–dense food cues [55–58]. Another study did not find any sex-based differences in the response to visual food cues (hedonic foods, neutral foods, and non-food objects) in the fed state; however, in the fasted state, there was higher activation in the nucleus accumbens and insula in women than in men with obesity [59]. In response to nutrition information graphics in a fasted state, both men and women with high BMI showed increased ACC activation compared to that in same-sex controls; additionally, women with high BMI showed increased insula activation and men with high BMI showed decreased PFC activation [60].
Female-specific studies reported associations between higher BMI and greater activation in reward and hedonic brain regions in response to visual food cues, under both fasted and fed states [61], in line with reduced neural responses to satiety [62]. A male-specific study showed that oxytocin administration attenuated functional connectivity between the ventral tegmental area (dopaminergic reward system) and the insula somatosensory cortex, amygdala, hippocampus, operculum, and middle temporal gyrus when viewing high-calorie foods [63].
Sex-Adjusted Studies
Sex-adjusted task-fMRI studies (n = 42) investigated responses to a wider range of stimuli (food and odor cues) and tasks (e.g., paradigms that focused on reward and executive function tasks) than direct-sex and single/within-sex studies. Studies using visual food-cue stimuli found greater obesity-related reactivity in the amygdala, insula, hippocampus, striatum, hypothalamus, ventral posterior cingulate, angular gyrus, putamen, and frontal cortex, including the dorsolateral PFC (in a fasting state) [64–67]; some of these regions show greater obesity-related reactivity in women based on the abovementioned direct-sex and single/within-sex findings [55–58].
No-Sex Studies
No-sex task-fMRI studies (n = 28) largely reported on the impact of interventions on reactivity to food-related cues [39, 68–85].
Structural Connectivity (DTI)
Direct-Sex and Single/Within-Sex Studies
Direct-sex and single/within-sex DTI studies (n = 3) reported on common and sex-specific associations between BMI/body fat and DTI measures of fractional anisotropy (FA), mean diffusivity (MD), and axial diffusivity (AD), as well as structural centrality. Specifically, high BMI/total body fat was associated with decreased AD in the corpus callosum [86] and increased global FA [10] in both men and women, while higher total body fat was negatively associated with global MD in women only [10]. A graph theory study reported greater structural centrality in the amygdala, hippocampus, and nucleus accumbens and anterior mid-cingulate cortex regions in women than in men with high BMI, while high BMI was associated with greater structural centrality in putamen and posterior insula regions in both men and women [87].
Sex-Adjusted Studies
Sex-adjusted studies (n = 23) mainly reported associations between obesity/high BMI/adiposity and FA. One study reported high BMI as related to a reduction in the global FA [88], opposite of that found in an abovementioned direct-sex and single/within-sex study [10]. Other studies reported obesity-related reductions in FA in widespread regions, including the bilateral frontal corticospinal tracts, right brainstem, uncinate fasciculus, corpus callosum, cerebral peduncle, internal capsule, corona radiata, superior and inferior longitudinal fasciculus, anterior and posterior thalamic radiation, external capsule, and superior cerebellar peduncle [89–91].
No-Sex Studies
No-sex DTI studies (n = 2) reported on the impact of exercise intervention on diffusivity parameters [42] and structural and functional connectivity parameters that best distinguished abdominal from non-abdominal obesity, indicating the importance of the frontoparietal and executive control networks [92].
Evolution of the Treatment of Sex During the Study Period
We evaluated the proportion of studies that considered sex as an important biological variable in the analyses (direct-sex and single/within-sex studies) published in early (2010–2015) and later (after 2015) portions of the study period. Ten (12.8%) of 78 studies published in the early study period and 16 (13.2%) of 121 studies published in the later study period considered sex as an important variable. Accordingly, the majority of studies that considered sex as an important variable were published in the late study period (16/26; 61.5%).
Keyword Co-occurrence Network
In the keyword co-occurrence network analysis of neuroimaging articles on adult obesity from 2010, three main clusters were identified: a reward/reactivity cluster, including keywords related to the extended reward system (e.g., striatum, dorsolateral PFC, amygdala) and reward-related functions (e.g., impulsivity, inhibitory control, decision-making), and brain reactivity to food-related cues and food intake; a disease-state cluster (e.g., dementia, insulin resistance, and Alzheimer’s disease); and a weight-loss intervention cluster (Fig. 3a). Although the keyword, sex differences, was included in, and well-connected with, the reward/reactivity cluster, it also had numerous links to the disease-state cluster, but did not show links with prominent intervention-related nodes (e.g. “weight loss,” “bariatric surgery”) (Fig. 3b).
Results of the keyword co-occurrence network analysis. Clusters of the top 100 keywords are indicated by different colors in the network visualization, including a reward/reactivity cluster in red, disease-state cluster in green, and weight-loss intervention cluster in blue. (a) The entire network is shown. (b) Keywords frequently co-occurring with the keyword, sex differences, are highlighted
Discussion
In this review, we categorized adult obesity neuroimaging studies according to the treatment of sex in the analyses and found that only ~ 13% of the studies treated sex as an important variable. There was a general lack of studies that examined sex differences, or at least presented disaggregated data, which could be useful in developing sex-specific biomarkers and interventions. Only 10 studies (5%) directly compared the sexes, and 16 (8%) limited the analysis to a single sex or analyzed the sexes separately, allowing qualitative comparisons, which may be a first step in understanding the impact of sex. This is in stark contrast to the 120 studies (60%) that controlled for sex and 53 studies (27%) that did not consider sex in the analysis, disallowing any comparisons. Moreover, the few single-sex studies were disproportionately focused on women rather than on men. This reveals the need for more research on sex differences and men in the field of obesity neuroimaging. In order to better understand and treat obesity, differences in obesity effects on the brain between the two sexes need to be elucidated, especially as the present review indicates that such differences do exist.
General Trends in Obesity Neuroimaging Findings According to Sex
The literature to date indicates that obesity is associated with numerous alterations in brain structure and responses to food-related stimuli; however, the extent and predominate regions involved differ between men and women with obesity, raising concerns regarding conclusions drawn from sex-adjusted and no-sex studies, as ignoring sex or adjusting for sex can mask important differences. Direct-sex studies generally showed more robust morphological alterations in subcortical and emotion-related regions in men [7–13] and more robust, widespread alterations in structural connectivity in women with obesity/high BMI/etc. [10, 87]. Additionally, women with obesity generally expressed increased reactivity to food-related cues in emotion-related regions, while men with obesity generally expressed increased reactivity to food-related cues in motor-related regions; this was especially true under fasting conditions [55–60].
Major Knowledge Gaps in the Field
Investigations of sex effects were especially lacking in intervention studies. This was clearly apparent in the keyword co-occurrence, as the keyword, sex differences, did not show any links with intervention-related keywords, such as bariatric surgery and weight loss. The keyword co-occurrence analysis revealed other major areas of research with limited consideration of sex differences, which included disease states such as dementia, Alzheimer’s disease, and insulin resistance. Many of the no-sex studies focused on the impact of various interventions on brain measures in the general obese population and in specific patient populations. Because sex was ignored, its relative contribution to differences in the neural response to treatment is unclear. However, non-imaging studies suggest sex differences in obesity intervention effectiveness [93], and the direct-sex studies in the current review suggest sex differences in reward-related and emotional-regulation region alterations under obese conditions. This suggests that obesity interventions should be tailored according to sex, as addressing specific neural alterations should enhance intervention efficacy. If more is known about how obesity differs between the two sexes, treatment for obesity can be optimized for both men and women; hence, there is need for more studies that use neuroimaging tools to explore sex differences and sex-specific obesity effects.
Limitations of This Review and the Field in General
Some of the studies reporting “sex-based” or “sex-specific” effects did not directly compare the sexes; rather, the researchers reported obesity-related effects in men and women considered separately. This approach (testing within each sex separately) can inflate the probability of concluding that a sex-specific effect is present compared to that when directly comparing the sexes [94]. In the present review, we coded such studies as single/within-sex, even though both sexes were included, as a direct comparison was lacking. This practice of testing within each sex separately may be unavoidable in typical small-sample neuroimaging studies that are underpowered for a sex interaction, but wish to provide information regarding potential sex effects. Many researchers have concerns about how to best conduct statistical analyses to assess the impact of sex in their dataset; a recent article provides an excellent conceptual guide [95•]. Additionally, partial least squares is a multivariate regression technique that is considered more sensitive than traditional neuroimaging analytic approach; as such, it may be better able to uncover sex differences [96]. Moreover, a focus on effect sizes, beyond p-values [95•, 97••], would help in understanding the scope of sex differences in the neural correlates of obesity and response to its treatment.
Concluding Remarks on Future Directions
The earliest articles covered in this review were published nearly a decade after the Institute of Medicine reported on the importance of considering sex in the exploration of the biological contributions to human health in 2001 [98]. Despite this, only ~ 13% of the studies reviewed treated sex as an important biological variable. However, the National Health Institutes first announced their intention to consider sex as a biological variable and established such policies in 2015/2016 (i.e., around the midpoint of the period covered by this review) to improve research rigor [99]. Additionally, journals have increasingly incorporated policies on the reporting of sex effects in their guidelines in recent years [100•]. Thus, additional pressure to consider sex as an important variable was in place during the later portion of the current review period. Accordingly, the majority of direct-sex and single/within-sex studies were published in later portion of the study period, after 2015 (16/26; 61.5%); unfortunately, this only slightly raised the proportion of direct-sex and single/within-sex studies from 12.8% in 2010–2015 to 13.2% since 2016. However, we hope that this trend will accelerate, enabling such studies to become the majority during the 2020s, filling in the abovementioned knowledge gaps, and improving our understanding of the neural correlates of obesity and response to its treatment in men and women, to aid in the optimization of treatment strategies. In the meantime, it is important to realize that a large proportion of the obesity neuroimaging literature informing future research has ignored the importance of sex.
Supplementary Materials: The full bibliography of reviewed articles according to the treatment of sex in the analysis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020:1–8.
Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. https://doi.org/10.1001/jama.286.10.1195.
Regensteiner JG, Reusch JEB. Sex Differences in cardiovascular consequences of hypertension, obesity, and diabetes: JACC Focus Seminar 4/7. J Am Coll Cardiol. 2022;79:1492–505. https://doi.org/10.1016/j.jacc.2022.02.010.
Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson Ch L, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O'Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. https://doi.org/10.1016/S0140-6736(16)30175-1.
Radhakrishnan S, Erbis S, Isaacs JA, Kamarthi S. Novel keyword co-occurrence network-based methods to foster systematic reviews of scientific literature. PLoS One. 2017;12:e0172778. https://doi.org/10.1371/journal.pone.0172778.
Noack A. Energy models for graph clustering. J Graph Algorithms Appl. 2007;11:453–80.
Huang Y, Li X, Jackson T, Chen S, Meng J, Qiu J, Chen H. Interaction effect of sex and body mass index on gray matter volume. Front Hum Neurosci. 2019;13:360. https://doi.org/10.3389/fnhum.2019.00360.
Arnoldussen IAC, Gustafson DR, Leijsen EMC, de Leeuw FE, Kiliaan AJ. Adiposity is related to cerebrovascular and brain volumetry outcomes in the RUN DMC study. Neurology. 2019;93:E864–78. https://doi.org/10.1212/wnl.0000000000008002.
Hayakawa YK, Sasaki H, Takao H, Yoshikawa T, Hayashi N, Mori H, Kunimatsu A, Aoki S, Ohtomo K. The relationship of waist circumference and body mass index to grey matter volume in community dwelling adults with mild obesity. Obes Sci Pract. 2018;4:97–105. https://doi.org/10.1002/osp4.145.
Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK biobank study. Radiology. 2019;291:763–71. https://doi.org/10.1148/radiol.2019181012.
Fehrenbach U, Jadan A, Auer TA, Kreutz K, Geisel D, Ziagaki A, Bobbert T, Wiener E. Obesity and pituitary gland volume – a correlation study using three-dimensional magnetic resonance imaging. Neuroradiology Journal. 2020;33:400–9. https://doi.org/10.1177/1971400920937843.
Kim H, Kim C, Seo SW, Na DL, Kim HJ, Kang M, Shin HY, Cho SK, Park SE, Lee J, Hwang JW, Jeon S, Lee JM, Kim GH, Cho H, Ye BS, Noh Y, Yoon CW, Guallar E. Association between body mass index and cortical thickness: among elderly cognitively normal men and women. Int Psychogeriatr. 2015;27:121–30. https://doi.org/10.1017/s1041610214001744.
Kim HJ, Kim C, Jeon S, Kang M, Kim YJ, Lee JM, Shin HY, Cho H, Ye BS, Kim JH, Jang EY, Cho J, Na DL, Rexrode KM, Seo SW. Association of body fat percentage and waist-hip ratio with brain cortical thickness: a study among 1777 cognitively normal subjects. Alzheimer Dis Assoc Disord. 2015;29:279–86. https://doi.org/10.1097/wad.0000000000000079.
Hempel R, Onopa R, Convit A. Type 2 diabetes affects hippocampus volume differentially in men and women. Diabetes Metab Res Rev. 2012;28:76–83. https://doi.org/10.1002/dmrr.1230.
Zsido RG, Heinrich M, Slavich GM, Beyer F, Masouleh SK, Kratzsch J, Raschpichler M, Mueller K, Scharrer U, Loffler M, Schroeter ML, Stumvoll M, Villringer A, Witte AV, Sacher J. Association of estradiol and visceral fat with structural brain networks and memory performance in adults. JAMA Netw Open. 2019;2:15. https://doi.org/10.1001/jamanetworkopen.2019.6126.
Coveleskie K, Gupta A, Kilpatrick LA, Mayer ED, Ashe-McNalley C, Stains J, Labus JS, Mayer EA. Altered functional connectivity within the central reward network in overweight and obese women. Nutr Diabetes. 2015;5:7. https://doi.org/10.1038/nutd.2014.45.
Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–64. https://doi.org/10.1002/hbm.20916.
Song LLT, Venkataraman K, Gluckman P, Chong YS, Chee MWL, Khoo CM, Leow MKS, Lee YS, Tai ES, Khoo EYH. Smaller size of high metabolic rate organs explains lower resting energy expenditure in Asian-Indian Than Chinese men. Int J Obes. 2016;40:633–8. https://doi.org/10.1038/ijo.2015.233.
Hamer M, Batty GD. Association of body mass index and waist-to-hip ratio with brain structure: UK Biobank study. Neurology. 2019;92:e594–600. https://doi.org/10.1212/wnl.0000000000006879.
Hidese S, Ota M, Matsuo J, Ishida I, Hiraishi M, Yoshida S, Noda T, Sato N, Teraishi T, Hattori K, Kunugi H. Association of obesity with cognitive function and brain structure in patients with major depressive disorder. J Affect Disord. 2018;225:188–94. https://doi.org/10.1016/j.jad.2017.08.028.
Honea RA, Szabo-Reed AN, Lepping RJ, Perea R, Breslin F, Martin LE, Brooks WM, Donnelly JE, Savage CR. Voxel-based morphometry reveals brain gray matter volume changes in successful dieters. Obesity. 2016;24:1842–8. https://doi.org/10.1002/oby.21551.
Janowitz D, Wittfeld K, Terock J, Freyberger HJ, Hegenscheid K, Volzke H, Habes M, Hosten N, Friedrich N, Nauck M, Domanska G, Grabe HJ. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage. 2015;122:149–57. https://doi.org/10.1016/j.neuroimage.2015.07.086.
Knight S, Laird E, O'Connor J, Newman L, Kenny RA. Central adiposity is associated with reduced cerebral perfusion: evidence from the Irish Longitudinal Study on Ageing (TILDA). Proc Nutr Soc. 2020;79. https://doi.org/10.1017/S0029665120000865.
Zhang Y, Ji G, Xu M, Cai W, Zhu Q, Qian L, Zhang YE, Yuan K, Liu J, Li Q, Cui G, Wang H, Zhao Q, Wu K, Fan D, Gold MS, Tian J, Tomasi D, Liu Y, Nie Y, Wang GJ. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes. 2016;40:1558–65. https://doi.org/10.1038/ijo.2016.98.
Brooks SJ, Benedict C, Burgos J, Kempton MJ, Kullberg J, Nordenskjöld R, Kilander L, Nylander R, Larsson EM, Johansson L, Ahlström H, Lind L, Schiöth HB. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obes. 2013;37:230–6. https://doi.org/10.1038/ijo.2012.13.
Weise CM, Bachmann T, Pleger B. Brain structural differences in monozygotic twins discordant for body mass index. Neuroimage. 2019;201. https://doi.org/10.1016/j.neuroimage.2019.07.019.
Cherbuin N, Sargent-Cox K, Fraser M, Sachdev P, Anstey KJ. Being overweight is associated with hippocampal atrophy: the PATH Through Life Study. Int J Obes. 2015;39:1509–14. https://doi.org/10.1038/ijo.2015.106.
Hsu FC, Yuan M, Bowden DW, Xu J, Smith SC, Wagenknecht LE, Langefeld CD, Divers J, Register TC, Carr JJ, Williamson JD, Sink KM, Maldjian JA, Freedman BI. Adiposity is inversely associated with hippocampal volume in African Americans and European Americans with diabetes. J Diabetes Complications. 2016;30:1506–12. https://doi.org/10.1016/j.jdiacomp.2016.08.012.
Beyer F, Masouleh SK, Kratzsch J, Schroeter ML, Röhr S, Riedel-Heller SG, Villringer A, Veronica Witte A. A metabolic obesity profile is associated with decreased gray matter volume in cognitively healthy older adults. Frontiers in Aging Neuroscience. 2019;10. https://doi.org/10.3389/fnagi.2019.00202.
Ambikairajah A, Tabatabaei-Jafari H, Walsh E, Hornberger M, Cherbuin N. Longitudinal changes in fat mass and the hippocampus. Obesity. 2020;28:1263–9. https://doi.org/10.1002/oby.22819.
Caunca MR, Gardener H, Simonetto M, Cheung YK, Alperin N, Yoshita M, DeCarli C, Elkind MSV, Sacco RL, Wright CB, Rundek T. Measures of obesity are associated with MRI markers of brain aging The Northern Manhattan Study. Neurology. 2019;93:E791–803. https://doi.org/10.1212/wnl.0000000000007966.
Hassenstab JJ, Sweet LH, Del Parigi A, McCaffery JM, Haley AP, Demos KE, Cohen RA, Wing RR. Cortical thickness of the cognitive control network in obesity and successful weight loss maintenance: a preliminary MRI study. Psychiatry Research - Neuroimaging. 2012;202:77–9. https://doi.org/10.1016/j.pscychresns.2011.09.008.
Bohon C, Garcia LC, Morton JM. Changes in cerebral cortical thickness related to weight loss following bariatric surgery. Obes Surg. 2018;28:2578–82. https://doi.org/10.1007/s11695-018-3317-6.
Bohon C, Geliebter A. Change in brain volume and cortical thickness after behavioral and surgical weight loss intervention. NeuroImage: Clinical. 2019;21. https://doi.org/10.1016/j.nicl.2018.101640.
Espeland MA, Erickson K, Neiberg RH, Jakicic JM, Wadden TA, Wing RR, Desiderio L, Erus G, Hsieh MK, Davatzikos C, Maschak-Carey BJ, Laurienti PJ, Demos-McDermott K, Nick Bryan R, Berkowitz RI, Bailey B, Bell Y, Butler N, Carvajal R, Davenport R, Diewald L, Elliott M, Faulconbridge L, Fields B, Huff K, Jones-Parker M, Keenan B, Leonard S, Li QY, Reilly K, Sexton K, Staley B, Voluck M, Wesche-Thobaben J, Hergenroeder A, Kurdilla S, Leckie RL, Mancino J, McGuire M, Murray T, Peluso A, Viszlay D, Watt JC, Egan C, Demos K, Annis K, Busha R, Damore C, Dunlap C, Fanella L, First L, Fisher M, Godbout S, Goldring A, Labossiere A, Bryan N, Nasrallah I, Bahnson J, Casanova R, Hayasaka S, Houston D, Lyday R, Barnes JM, Beckner TD, Cook D, Gordon M, Hege D, Hodges A, Hogan P, Morgan A, Pate G, Walker J. Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care. 2016;39:764–71. https://doi.org/10.2337/dc15-2230.
Wang Y, Ji G, Hu Y, Li G, Ding Y, Hu C, Liu L, Zhang W, von Deneen KM, Han Y, Cui G, Wang H, Manza P, Volkow ND, Nie Y, Wang GJ, Zhang Y. Laparoscopic sleeve gastrectomy induces sustained changes in gray and white matter brain volumes and resting functional connectivity in obese patients. Surgery for Obesity and Related Diseases. 2020;16:1–9. https://doi.org/10.1016/j.soard.2019.09.074.
Prehn K, Profitlich T, Rangus I, Heßler S, Witte AV, Grittner U, Ordemann J, Flöel A. Bariatric surgery and brain health—a longitudinal observational study investigating the effect of surgery on cognitive function and gray matter volume. Nutrients. 2020;12. https://doi.org/10.3390/nu12010127.
Tuulari JJ, Karlsson HK, Antikainen O, Hirvonen J, Pham T, Salminen P, Helmio M, Parkkola R, Nuutila P, Nummenmaa L. Bariatric surgery induces white and grey matter density recovery in the morbidly obese: a voxel-based morphometric study. Hum Brain Mapp. 2016;37:3745–56. https://doi.org/10.1002/hbm.23272.
Deckersbach T, Das SK, Urban LE, Salinardi T, Batra P, Rodman AM, Arulpragasam AR, Dougherty DD, Roberts SB. Pilot randomized trial demonstrating reversal of obesity-related abnormalities in reward system responsivity to food cues with a behavioral intervention. Nutrition and Diabetes. 2014;4. https://doi.org/10.1038/nutd.2014.26.
Rullmann M, Preusser S, Poppitz S, Heba S, Gousias K, Hoyer J, Schütz T, Dietrich A, Müller K, Hankir MK, Pleger B. Adiposity related brain plasticity induced by bariatric surgery. Frontiers in Human Neuroscience. 2019;13. https://doi.org/10.3389/fnhum.2019.00290.
Rullmann M, Preusser S, Poppitz S, Heba S, Hoyer J, Schütz T, Dietrich A, Müller K, Pleger B. Gastric-bypass surgery induced widespread neural plasticity of the obese human brain. Neuroimage. 2018;172:853–63. https://doi.org/10.1016/j.neuroimage.2017.10.062.
Mueller K, Moller HE, Horstmann A, Busse F, Lepsien J, Bluher M, Stumvoll M, Villringer A, Pleger B. Physical exercise in overweight to obese individuals induces metabolic-and neurotrophic-related structural brain plasticity. Frontiers in Human Neuroscience. 2015;9. https://doi.org/10.3389/fnhum.2015.00372.
Osadchiy V, Mayer EA, Bhatt R, Labus JS, Gao L, Kilpatrick LA, Liu C, Tillisch K, Naliboff B, Chang L, Gupta A. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes Sci Pract. 2019;5:416–36. https://doi.org/10.1002/osp4.362.
Gupta A, Mayer EA, Labus JS, Bhatt RR, Ju T, Love A, Bal A, Tillisch K, Naliboff B, Sanmiguel CP, Kilpatrick LA. Sex commonalities and differences in obesity-related alterations in intrinsic brain activity and connectivity. Obesity (Silver Spring). 2018;26:340–50. https://doi.org/10.1002/oby.22060.
Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson EM, Sundbom M, Benedict C, Schioth HB. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes. 2016;40:1687–92. https://doi.org/10.1038/ijo.2016.105.
Contreras-Rodríguez O, Martín-Pérez C, Vilar-López R, Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: link to food craving and weight gain. Biol Psychiat. 2017;81:789–96. https://doi.org/10.1016/j.biopsych.2015.11.020.
Contreras-Rodríguez O, Vilar-López R, Andrews ZB, Navas JF, Soriano-Mas C, Verdejo-García A. Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci Rep. 2017;7:9951. https://doi.org/10.1038/s41598-017-09874-y.
Contreras-Rodriguez O, Burrows T, Pursey KM, Stanwell P, Parkes L, Soriano-Mas C, Verdejo-Garcia A. Food addiction linked to changes in ventral striatum functional connectivity between fasting and satiety. Appetite. 2019;133:18–23. https://doi.org/10.1016/j.appet.2018.10.009.
Le TM, Liao DL, Ide J, Zhang S, Zhornitsky S, Wang W, Li CSR. The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int J Obes. 2020;44:1097–107. https://doi.org/10.1038/s41366-019-0496-8.
Cerit H, Davidson P, Hye T, Moondra P, Haimovici F, Sogg S, Shikora S, Goldstein JM, Evins AE, Whitfield-Gabrieli S, Stoeckel LE, Holsen LM. Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity. 2019;27:1846–1855. https://doi.org/10.1002/oby.22607.
Kim SH, Park BY, Byeon K, Park H, Kim Y, Eun YM, Chung JH. The effects of high-frequency repetitive transcranial magnetic stimulation on resting-state functional connectivity in obese adults. Diabetes Obes Metab. 2019;21:1956–66. https://doi.org/10.1111/dom.13763.
Lepping RJ, Bruce AS, Francisco A, Yeh HW, Martin LE, Powell JN, Hancock L, Patrician TM, Breslin FJ, Selim N, Donnelly JE, Brooks WM, Savage CR, Simmons WK, Bruce JM. Resting-state brain connectivity after surgical and behavioral weight loss. Obesity. 2015;23:1422–8. https://doi.org/10.1002/oby.21119.
McDermott KD, Williams SE, Espeland MA, Erickson K, Neiberg R, Wadden TA, Bryan RN, Desiderio L, Leckie RL, Falconbridge LH, Jakicic JM, Alonso-Alonso M, Wing RR. Impact of intensive lifestyle intervention on neural food cue reactivity: action for Health in Diabetes Brain Ancillary Study. Obesity. 2019;27:1076–84. https://doi.org/10.1002/oby.22496.
Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS ONE. 2013;8. https://doi.org/10.1371/journal.pone.0059114.
Atalayer D, Pantazatos SP, Gibson CD, McOuatt H, Puma L, Astbury NM, Geliebter A. Sexually dimorphic functional connectivity in response to high vs. low energy-dense food cues in obese humans: an fMRI study. Neuroimage. 2014;100:405–13. https://doi.org/10.1016/j.neuroimage.2014.05.054.
Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–6. https://doi.org/10.1016/j.bbr.2012.12.023.
Killgore WDS, Weber M, Schwab ZJ, Kipman M, DelDonno SR, Webb CA, Rauch SL. Cortico-limbic responsiveness to high-calorie food images predicts weight status among women. Int J Obes. 2013;37:1435–42. https://doi.org/10.1038/ijo.2013.26.
Sayer RD, Tamer GG, Chen NN, Tregellas JR, Cornier MA, Kareken DA, Talavage TM, McCrory MA, Campbell WW. Reproducibility assessment of brain responses to visual food stimuli in adults with overweight and obesity. Obesity. 2016;24:2057–63. https://doi.org/10.1002/oby.21603.
Legget KT, Cornier MA, Bessesen DH, Mohl B, Thomas EA, Tregellas JR. Greater reward-related neuronal response to hedonic foods in women compared with men. Obesity (Silver Spring). 2018;26:362–7. https://doi.org/10.1002/oby.22082.
Zerbini C, Luceri B, Marchetti A, Di Dio C. Shaping consumption propensity through the emotional response evoked by nutritional labels: evidence from an fMRI study. Food research international (Ottawa, Ont.). 2019;125:108547. https://doi.org/10.1016/j.foodres.2019.108547.
Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A. Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imagining taste: an event-related fMRI study. Int J Obes. 2012;36:627–37. https://doi.org/10.1038/ijo.2011.213.
Gobbi S, Weber SC, Graf G, Hinz D, Asarian L, Geary N, Leeners B, Hare TA, Tobler PN. Reduced neural satiety responses in women affected by obesity. Neuroscience. 2020;447:94–112. https://doi.org/10.1016/j.neuroscience.2020.07.022.
Kerem L, Hadjikhani N, Holsen L, Lawson EA, Plessow F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int J Obes. 2020;44:980–9. https://doi.org/10.1038/s41366-019-0489-7.
Eldor R, Daniele G, Huerta C, Al-Atrash M, Adams J, De Fronzo R, Duong T, Lancaster J, Zirie M, Jayyousi A, Abdul-Ghani M. Discordance between central (brain) and pancreatic action of exenatide in lean and obese subjects. Diabetes Care. 2016;39:1804–10. https://doi.org/10.2337/dc15-2706.
Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 2013;36:394–402. https://doi.org/10.2337/dc12-1112.
Ho A, Kennedy J, Dimitropoulos A. Neural correlates to food-related behavior in normal-weight and overweight/obese participants. PLoS ONE. 2012;7. https://doi.org/10.1371/journal.pone.0045403.
Contreras-Rodriguez O, Mata F, Verdejo-Román J, Ramírez-Bernabé R, Moreno D, Vilar-Lopez R, Soriano-Mas C, Verdejo-García A. Neural-based valuation of functional foods among lean and obese individuals. Nutr Res. 2020;78:27–35. https://doi.org/10.1016/j.nutres.2020.03.006.
Sayer RD, Amankwaah AF, Tamer GG, Jr., Chen N, Wright AJ, Tregellas JR, Cornier MA, Kareken DA, Talavage TM, McCrory MA, Campbell WW. Effects of dietary protein and fiber at breakfast on appetite, ad libitum energy intake at lunch, and neural responses to visual food stimuli in overweight adults. Nutrients. 2016;8. https://doi.org/10.3390/nu8010021.
Sayer RD, Dhillon J, Tamer GG, Cornier MA, Chen NN, Wright AJ, Campbell WW, Mattes RD. Consuming almonds vs. isoenergetic baked food does not differentially influence postprandial appetite or neural reward responses to visual food stimuli. Nutrients. 2017;9:11. https://doi.org/10.3390/nu9080807.
Szabo-Reed AN, Martin LE, Hu J, Yeh HW, Powell J, Lepping RJ, Patrician TM, Breslin FJ, Donnelly JE, Savage CR. Modeling interactions between brain function, diet adherence behaviors, and weight loss success. Obes Sci Pract. 2020;6:282–92. https://doi.org/10.1002/osp4.403.
Bruce AS, Bruce JM, Ness AR, Lepping RJ, Malley S, Hancock L, Powell J, Patrician TM, Breslin FJ, Martin LE, Donnelly JE, Brooks WM, Savage CR. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity. 2014;22:337–43. https://doi.org/10.1002/oby.20630.
Murdaugh DL, Cox JE, Cook EW, Weller RE. FMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–21. https://doi.org/10.1016/j.neuroimage.2011.10.071.
Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105:1028–34. https://doi.org/10.1016/j.physbeh.2011.11.023.
Neseliler S, Hu W, Larcher K, Zacchia M, Dadar M, Scala SG, Lamarche M, Zeighami Y, Stotland SC, Larocque M, Marliss EB, Dagher A. Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metab. 2019;29:39-49.e4. https://doi.org/10.1016/j.cmet.2018.09.024.
Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, De Wit S, Nathan PJ, Brooke A, O’Rahilly S, Farooqi IS, Bullmore ET. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci. 2010;30:14346–55. https://doi.org/10.1523/JNEUROSCI.3323-10.2010.
Ten Kulve JS, Veltman DJ, Van Bloemendaal L, Groot PFC, Ruhé HG, Barkhof F, Diamant M, Ijzerman RG. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol. 2016;229:1–12. https://doi.org/10.1530/JOE-15-0461.
Van Bloemendaal L, Ijzerman RG, Ten Kulve JS, Barkhof F, Veltman DJ, Diamant M. Exenatide blunts the increases in CNS reward and satiety activation by visual food-related stimuli in obese individuals. Diabetes. 2013;62:A542. https://doi.org/10.2337/db13-1825-2160.
Ten Kulve JS, Veltman DJ, Van Bloemendaal L, Barkhof F, Drent ML, Diamant M, Ijzerman RG. Liraglutide reduces CNS activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care. 2016;39:214–21. https://doi.org/10.2337/dc15-0772.
Moser VC, Phillips PM, Hedge JM, McDaniel KL. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP). Neurotoxicol Teratol. 2015;52:236–47. https://doi.org/10.1016/j.ntt.2015.08.004.
Farr OM, Upadhyay J, Gavrieli A, Camp M, Spyrou N, Kaye H, Mathew H, Vamvini M, Koniaris A, Kilim H, Srnka A, Migdal A, Mantzoros CS. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled, double-blind clinical trial. Diabetes. 2016;65:2943–53. https://doi.org/10.2337/db16-0635.
Farr OM, Upadhyay J, Rutagengwa C, DiPrisco B, Ranta Z, Adra A, Bapatla N, Douglas VP, Douglas KAA, Nolen-Doerr E, Mathew H, Mantzoros CS. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes Metab. 2019;21:2459–64. https://doi.org/10.1111/dom.13827.
Kahathuduwa CN, Davis T, O’Boyle M, Binks M. Do scores on the Food Craving Inventory and Three-Factor Eating Questionnaire correlate with expected brain regions of interest in people with obesity? Physiol Behav. 2018;188:1–10. https://doi.org/10.1016/j.physbeh.2018.01.018.
Kahathuduwa CN, Davis T, O'Boyle M, Boyd LA, Chin SH, Paniukov D, Binks M. Effects of 3-week total meal replacement vs. typical food-based diet on human brain functional magnetic resonance imaging food-cue reactivity and functional connectivity in people with obesity. Appetite. 2018;120:431–441. https://doi.org/10.1016/j.appet.2017.09.025.
Kohl SH, Veit R, Spetter MS, Günther A, Rina A, Lührs M, Birbaumer N, Preissl H, Hallschmid M. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: a randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609. https://doi.org/10.1016/j.neuroimage.2019.02.033.
Drummen M, Dorenbos E, Vreugdenhil ACE, Stratton G, Raben A, Westerterp-Plantenga MS, Adam TC. Associations of brain reactivity to food cues with weight loss, protein intake and dietary restraint during the PREVIEW intervention. Nutrients. 2018;10. https://doi.org/10.3390/nu10111771.
Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS One. 2011;6:e18544. https://doi.org/10.1371/journal.pone.0018544.
Gupta A, Mayer EA, Hamadani K, Bhatt R, Fling C, Alaverdyan M, Torgerson C, Ashe-McNalley C, Van Horn JD, Naliboff B, Tillisch K, Sanmiguel CP, Labus JS. Sex differences in the influence of body mass index on anatomical architecture of brain networks. Int J Obes. 2017;41:1185–95. https://doi.org/10.1038/ijo.2017.86.
Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74:682–90. https://doi.org/10.1097/PSY.0b013e318261909c.
Lou B, Chen M, Luo X, Dai Y. Reduced right frontal fractional anisotropy correlated with early elevated plasma LDL levels in obese young adults. PLoS ONE. 2014;9. https://doi.org/10.1371/journal.pone.0108180.
Zhang R, Beyer F, Lampe L, Luck T, Riedel-Heller SG, Loeffler M, Schroeter ML, Stumvoll M, Villringer A, Witte AV. White matter microstructural variability mediates the relation between obesity and cognition in healthy adults. Neuroimage. 2018;172:239–49. https://doi.org/10.1016/j.neuroimage.2018.01.028.
Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, Heaps JM, Salminen LE, Baker LM, Scott SE, Cooley SA, Gunstad J, Paul RH. Brain structure and cognitive correlates of body mass index in healthy older adults. Behav Brain Res. 2015;278:342–7. https://doi.org/10.1016/j.bbr.2014.10.010.
Park BY, Lee MJ, Kim M, Kim SH, Park H. Structural and functional brain connectivity changes between people with abdominal and non-abdominal obesity and their association with behaviors of eating disorders. Front Neurosci. 2018;12:13. https://doi.org/10.3389/fnins.2018.00741.
Sharkey T, Whatnall MC, Hutchesson MJ, Haslam RL, Bezzina A, Collins CE, Ashton LM. Effectiveness of gender-targeted versus gender-neutral interventions aimed at improving dietary intake, physical activity and/or overweight/obesity in young adults (aged 17–35 years): a systematic review and meta-analysis. Nutr J. 2020;19:78. https://doi.org/10.1186/s12937-020-00594-0.
Garcia-Sifuentes Y, Maney DL. Reporting and misreporting of sex differences in the biological sciences. Elife. 2021;10. https://doi.org/10.7554/eLife.70817.
• Beltz AM, Beery AK, Becker JB. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology. 2019;44:2155–8. https://doi.org/10.1038/s41386-019-0524-3. Authors provide a conceptual guide to the statistical analysis of sex differences.
McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–63. https://doi.org/10.1016/j.neuroimage.2004.07.020.
•• Vandekar SN, Stephens J. Improving the replicability of neuroimaging findings by thresholding effect sizes instead of p-values. Hum Brain Mapp. 2021;42:2393–8. https://doi.org/10.1002/hbm.25374. Authors provide a compelling argument for shifting the focus to effect sizes rather than p-values, which could not only improve neuroimaging replicability, but could provide a better understanding of the degree of sex effects.
Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences; Wizemann TP, ML, ed. Exploring the Biological Contributions to Human Health: Does Sex Matter? 2001, National Academies Press (US): Washington (DC).
Health NIo, NOT-OD-15–102: consideration of sex as a biological variable in NIH-funded Research. 2015.
• Raising the bar on sex and gender reporting in research. Nat Commun. 2022;13:2845. https://doi.org/10.1038/s41467-022-30398-1. This paper provides updated guidance on the reporting of sex and gender.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kilpatrick, L.A., An, H.M., Pawar, S. et al. Neuroimaging Investigations of Obesity: a Review of the Treatment of Sex from 2010. Curr Obes Rep 12, 163–174 (2023). https://doi.org/10.1007/s13679-023-00498-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00498-0