Abstract
Atopic dermatitis is an exceedingly common condition that is often difficult to treat. In this review, the pathophysiology and recent developments are discussed to understand how to better treat atopic dermatitis through the improved understanding of the breakdown of the skin barrier in atopic patients. The available treatments that address these barrier defects are discussed in the light of recent publications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis affects 15 % to 30 % of children and 2 % to 10 % of adults of industrialized countries—rates that are two to three times higher than those of 30 years ago. The vast majority of atopic dermatitis cases onset early in life: 45 % by 6 months, 60 % by 1 year, and 85 % by 5 years [1]. Ichthyosis vulgaris is the most common inherited disease of keratinization. Current understanding of filaggrin—the gene implicated in ichthyosis vulgaris and, more recently, atopic dermatitis—has genetic implications that have drastically advanced the understanding of the pathophysiology of atopic dermatitis over the last 26 years. In 1985, ichythyosis vulgaris was found to be associated with decreased expression of filaggrin; subsequently, in 2006, Smith et al. [2•] discovered that loss-of-function mutations caused ichthyosis vulgaris and an increased risk for atopic eczema.
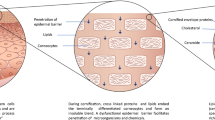
The lipids of the stratum corneum serve to retain water as a barrier to permeability. This barrier is made up of several components, including corneocytes and their surrounding mature lamellar bodies filled with ceramides, cholesterol, and fatty acids. These hydrophobic molecules in the skin provide a means to prevent the loss of moisture that results in the breakdown of the epidermis, superinfection by opportunistic organisms, and increased antigen exposure. Ceramides, in addition to free fatty acids and cholesterol, are lipids found in the stratum corneum, of which ceramides account for half of the lipid weight [3]. More specifically, ceramides are amide-linked fatty acids that are produced via hydrolysis of glucosylceramide and of sphingomyelin or produced by the enzyme serine palmitoyltransferase. The stratum corneum contains at least nine ceramides [4], and a decrease in the concentration of these ceramides within the stratum corneum is implicated as a cause of compromised integrity, as in atopic dermatitis. A phenomenon relating filaggrin and ceramides is observed in subjects with a mutation in filaggrin that alters the ceramide ratio within the stratum corneum to create a lower overall quantity of ceramides [5].
Current Perspectives
In 2009, genetic defects in filaggrin expression were found to allow increased transepidermal sensitization across the skin of mice. Most recently, in 2011, filaggrin deficiency was associated with peanut allergy and filaggrin size variation with a risk of eczema. Within the filaggrin gene sequence, a greater copy number variation (which translates to an increased size of the filaggrin gene) appears to be protective, whereas a smaller gene size appears to be predictiveof eczema development. Seemingly a dose-dependent relationship exists between the size of filaggrin gene and protection against acquiring eczema [6].
Filaggrin and its precursor, profilaggrin, have numerous roles in maintaining the integrity of an optimal skin barrier [7]. Profilaggrin may regulate terminal epidermal differentiation [8]. In particular, the process of nuclei expulsion of keratinocytes within the stratum corneum may involve profilaggrin [9]. Filaggrin is involved in the production of squames by binding to keratins and intermediate filaments within keratinocytes, which allows for tight bundle formation and resultant compaction within the stratum corneum outer layer [10]. Although filaggrin is not required for this flattening process, loss-of-function mutations in filaggrin appear to result in a disorganized cytoskeletal matrix by several mechanisms [11]. Filaggrin is also necessary for the process that protects the skin against water loss and microorganism entry, by participating in the protein-lipid cell layer of keratinocytes [12].
Even the degradation products of filaggrin have protective barrier function, as the resultant amino acids create a hygroscopic layer that retains moisture and is thus known as the “natural moisturizing factor” [13]. Filaggrin-deficient skin has a decreased concentration of these amino acids and subsequent increase in water loss from the epidermis [14]. Metabolites of filaggrin, primarily the histidine-derived products, create an acidic surface to the epidermis that is antimicrobial in nature. Filaggrin-deficient skin results in an increased pH [5]. Miajilovic et al. [15] demonstrated that Staphylococcus aureus growth is hindered at physiologic concentrations of filaggrin breakdown products. Studies in 2010 revealed that an acidic pH of the epidermis is also essential for enzyme activation in the processing of ceramide [16]. Homozygous absence of the filaggrin gene is associated with ichthyosis vulgaris, whereas “haploinsufficiency,” or heterozygotes for the filaggrin loss-of-function mutation, manifests commonly as keratosis pilaris and/or palmoplantar hyperlinearity [17]. The association of eczema with various atopic diseases, including asthma and allergies, has been well established. In recent years, loss-of-function filaggrin mutations have been linked to eczema in 2006 [18] and each of these conditions: allergic sensitization in 2009 [19], eczema-associated asthma in 2008 [20], allergic rhinitis in 2009 [21], and peanut allergy in 2011 [22•]. Brown and McLean [23] emphasize that the predisposition common among these atopic diseases is a defect in the skin barrier.
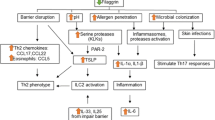
De Benedetto et al. [24] explain that mutations in the filaggrin gene alone are insufficient to cause allergen sensitization but predispose susceptible patients to such “cutaneous reactivity” in the presence of additional genetic and environmental factors. Filaggrin does not appear to have a prominent role in transepidermal water loss (TEWL), a well-described benchmark of atopic dermatitis. TEWL parallels the severity of atopic dermatitis, but the mechanism is unclear presently. Thus, additional genetic defects likely exist to fully explain the pathophysiology of atopic dermatitis. Genetic defects of barrier function may include either stratum corneum, tight junctions, or both, and the “relative contribution of each may contribute to the heterogeneity characteristic” of atopic dermatitis, including qualities like “disease onset, natural history, magnitude of allergic sensitization, and comorbid atopic condition” [24], p. 955]. Following skin barrier compromise, various epidermal-derived cytokines have been implicated in promoting a T helper type 2 (Th2) response, including thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33. Additionally, skin barrier compromise appears to promote the migration of Langerhan cells to the epidermis via tight junctions in order to take up antigens [25].
IL-31 was discovered and named in 2004, after its receptor was found to cause pruritus, airway hypersensitivity, hair loss, and skin lesions upon activation [26]. Takaoka et al. [27] used an experimental animal model of atopic dermatitis (the NC/Nga mouse) to demonstrate that IL-31 is the cytokine likely involved in causing pruritus, a well recognized and significant symptom of eczema. The expression of IL-31 in the skin of atopic dermatitis models positively correlated to scratching counts (r 2 = 0.89, p = <0.0006), inflammation score (r 2 = 0.73, p = <.0.0033), and slightly to TEWL (r 2 = 0.56, p = <0.0936) [27]. Subsequently, Baron et al. [28] pursued these findings in humans and sought to establish the pruritogenic factor in individuals with atopic dermatitis. IL-31 is a cytokine released by CD4 T cells, mast cells, and monocytes. IL-31 was found in higher concentrations in atopic dermatitis biopsies, using immunohistochemical staining, when compared to other itching diseases and other Th2-mediated diseases; however, there was not a strong relationship between IL-31 and pruritus. This suggests that IL-31 may be more specific in the pathogenesis of atopic dermatitis than other dermatologic conditions and potentially a target for future therapy [28•].
The management of eczema is stepwise. First and foremost, the focus should be maintaining the integrity of skin barrier with emollients and adequate hygiene. Topical corticosteroids are first-line for an eczema flare, with topical calcineurin inhibitors being recommended by the Food and Drug Administration (FDA) as second-line agents for moderate-to-severe eczema. When eczema becomes secondarily infected, oral antibiotics may be started. Of note, eczema herpeticum (eczema secondarily infected by herpes simplex virus) may require the urgent hospitalization of the patient for further care, including intravenous antiviral therapy [29]. The vast majority of eczema therapies are topical, and most recent advances have been designed to aid in repairing the barrier defect of atopic skin. Calcineurin inhibitors, like tacrolimus and pimecrolimus, help control pruritus and disease activity, and can be used to reduce time between disease flares. Bath emollients, dilute bleach baths, and occlusive treatments, although widely used, have been lacking in terms of large, powerful studies and randomized controlled trials [30], but the widespread use of these interventions may be helpful to patients suffering with atopic dermatitis.
Recent Advancements in Barrier Protection
Medicated device creams and foams have been the most recent therapy introduced for the treatment of eczema. These have several roles in countering atopic dermatitis, including restoration of the disrupted lipid content of atopic skin, and anti-inflammation and antioxidant properties [31]. Schmitt et al. [32] conducted a systematic review and meta-analysis of nine randomized controlled trials to evaluate the efficacy and tolerability of topical corticosteroids and/or topical calcineurin inhibitors for the prevention of flares in atopic eczema patients. Draelos [33•] evaluated the efficacy of ceramide-containing hyaluronic acid-based foam (Hylatopic; Onset Therapeutics, Cumberland, RI, USA) compared with ceramide-containing emulsion cream (EpiCeram; PuraCap Pharmaceutical, South Plainfield, NJ, USA) for the treatment of mild-to-moderate atopic dermatitis in a double-blinded study of 20 subjects with at least 10 % body surface area and symmetric involvement. These subjects were assigned randomly to use a ceramide-containing foam on one side of their body and a ceramide-containing emulsion cream on the other. At baseline, week 2, and week 4, the subjects and investigator assessed erythema, scaling, lichenification, excoriation, itching, stinging, and burning. The subjects and investigators used a six-point scale to assess the severity of disease (0 = no disease; 5 = severe). The subjects also assessed which product they preferred. The hyaluronic acid foam lead to statistically significant improvement in the severity score of atopic dermatitis at both weeks 2 and week 4 (p = 0.016 and <0.001, respectively), whereas the ceramide cream produced statistically significant improvement at 4 weeks (p < 0.001), but not 2 weeks (p = 0.155). The change from baseline for the foam group was a 26.9 % reduction in severity at week 2 and 62.3 % at week 4, whereas the cream resulted in a 17.9 % reduction in severity at week 2 and 47.3 % at week 4. No subjects experienced adverse reactions to either formulation.
In nine aesthetic categories (prefer to continue using, more willing to spend co-pay on, worked better, less odor, rubs into skin easier, easier to use, more soothing, more moisturizing, spreads more easily), the foam was preferred by the subjects, with rates ranging from 56 % to 78 % as compared to rates of 22 % to 44 % for the cream [33•]. Strengths of this study include its double-blinded and randomized design. Plus, having subjects serve as both a case and control eliminated confounding factors relating to individual variation of application technique and of disease responsiveness. This application technique could have introduced error into the study, as subjects could confuse which cream for which location; however, an attempt to control this was made with the compliance diary. Limitations of the study include the following: only female subjects, subjects were all over 18 years old (atopic dermatitis is most prevalent among children), one clinical research center, small sample size, and rather subjective evaluation of disease and response. Draelos [33•] noted that, in addition to the superior response of eczema concluded here, hyaluronic acid foam may also result in increased compliance among patients, secondary to its ease of administration and aesthetic benefits. The authors conclude that, in managing atopic dermatitis, hyaluronic acid emollient foams may be useful in the treatment phase in conjunction with other topical agents (ie, topical corticosteroids) or in the maintenance phase as monotherapy [33•].
Miller et al. [34] conducted a randomized, prospective trial of head-to-head comparisons of two prescription device moisturizers with an over-the-counter (OTC) moisturizer in monotherapy for pediatric patients with atopic dermatitis of mild-to-moderate severity. This was the first study to evaluate the efficacy of prescription versus OTC on the basis of clinical results and cost. Three treatment arms were organized, with 13 subjects in each: 1) barrier repair cream with glycyrrhetinic acid, or BRC-Gly (Atopiclair; Sinclair IS Pharma, London, UK); 2) barrier repair predominantly with ceramide, or BRC-Cer (EpiCeram, PuraCap Pharmaceutical, South Plainfield, NJ, USA); and 3) a petroleum-based OTC skin-protectant moisturizer, or OTC-Pet (Aquaphor; Beiersdorf, Wilton, CT, USA). At the initial visit, the severity of atopic dermatitis was graded on a five-point scale (the Investigator’s Global Assessment [IGA] severity scale). The 39 subjects were 2 to 17 years old with mild-to-moderate atopic dermatitis (scores of 2 to 3) and at least 1 % body surface involvement. The subjects were given identical instructions of applying their assigned cream three times daily for 3 weeks and were evaluated at baseline, day 7, and day 21.
Various scales were used at follow-up visits to individually assess disease severity, body surface involvement, global improvement, and itch intensity. Compliance did not vary between treatment groups, and a statistically significant correlation was found between “better reported usage” and “better improvement in pruritus” at day 7 and day 21 (p = 0.04 and p = 0.006, respectively). Better reported usage did significantly correlate with any other assessment value. Overall, only the OTC-Pet group showed statistically significant improvement in all four areas: IGA, Eczema Area and Severity Index (EASI), Visual Analog Scale (VAS) for itch intensity, and body surface area (BSA) involvement (p < 0.05). At day 7, the OTC-Pet group had the greatest median percent improvement in EASI (46 %), VAS (32 %), and BSA (33 %), and again at day 21 the highest in EASI (64 %) and BSA (33 %). No statistical difference, however, could be measured between the three treatment arms, indicating similar efficacy. Relative cost-effectiveness ratio (CER) was calculated using the following formula: \( {\text{CER}} = {{{\left( {\$ {\text{cost}}\;{\text{of}}\;{\text{intervention}} - \$ {\text{cost}}\;{\text{of}}\;{\text{comparator}}} \right)}} \left/ {{\left( {{\text{effect}}\;{\text{of}}\;{\text{intervention}} - {\text{effect}}\;{\text{of}}\;{\text{comparator}}} \right)}} \right.} \) [34]. The cost-efficacy of a product was determined by the cost per improvement in EASI score. The cost-efficacy of BRC-Gly, BRC-Cer, and OTC-Pet was $2.82, $2.35, and $0.05, respectively; the authors calculated OTC-Pet as 47 times more cost-effective than the BRCs. Miller et al. [34] concluded that OTC-Pet has a large advantage compared with prescription devices because of its apparent similar effectiveness in controlling disease and profound cost savings demonstrated in their study. Limitations of the study include utilizing data from all 39 subjects despite 5 straying from study protocol, small sample size, and a small body surface area involvement minimum to be eligible for study enrollment [35].
Jensen et al. [36•]. evaluated the gene expression of atopic dermatitis lesions treated with topical pimecrolimus and betamethasone to gain insight regarding the mechanism by which these drugs treat atopic dermatitis. Jensen et al. [37] found in 2009 that betamethasone valerate was clinically more efficient than pimecrolimus in resolving symptoms but impaired skin barrier repair and caused atrophy; however, pimecrolimus did not cause these side effects, suggesting that it is better suited than betamethasone valerate for long-term therapy of atopic dermatitis. After analysis of the expression profiles of genes involved in inflammation and immune response in lesions of atopic dermatitis, the investigators found significantly decreased mRNA levels of numerous inflammatory cells (specifically CD1a, CD11b, and CD11c cells and T-cell markers) in betamethasone-treated skin compared with pimecrolimus. Chemotactic factors (the C-C motif of chemokine ligand [CCL2, CCL19, CCL26] and E-selectin), elafin, metalloproteinase 12, and serine peptidase inhibitors (B3 and B4) were decreased in in both treatment groups, although the effect was greater in the betamethasone group than pimecrolimus. Tumor necrosis factor receptor superfamily and transforming growth factor-beta 1 expression was slightly decreased in both groups. After analysis of expression profiles of genes involved with dermal and epidermal integrity, the investigators found a decrease in expression of various collagen-encoding genes in skin treated with betamethasone, but not with pimecrolimus. Expression of keratin variants relating to epidermal differentiation was amplified after betamethasone therapy and, to a lesser extent, after pimecrolimus. Keratin variants relating to inflammation and proliferation were greatly reduced by betamethasone and, to a lesser extent, pimecrolimus. Expression of filaggrin and small proline-rich-like molecules (late markers of epidermal differentiation) was slightly decreased in both treatment groups. Genes involved with lipid metabolism showed varying degrees of change in both treatment groups. This study demonstrated that both pimecrolimus and betamethasone affected keratinocyte and epidermal differentiation, immune system response, and production of cytokines. Pimecrolimus also affected expression of genes employed in cell proliferation and homeostasis, whereas betamethasone affected additional genes of epidermal inflammation, chemotaxis, and immune response. In conclusion, this study confirms the notion of using corticosteroids for acute flares in atopic dermatitis, as they profoundly improve the disease on a molecular basis; however, calcineurin inhibitors may be more appropriate for long-term maintenance than corticosteroids, since these medications are not associated with atrophy or loss of structural integrity of the skin [36•].
Trookman et al. [38•] compared the efficacy of desonide hydrogel 0.05 % (Desonate Gel; Intendis, Morristown, NJ, USA) with generic desonide ointment 0.05 % (Fougera & Co., Melville, New York, USA) in treating mild-to-moderate atopic dermatitis in a randomized, investigator-blinded, parallel-group, controlled trial. Forty-four subjects over 12 years old with mild-to-moderate atopic dermatitis were assigned to either the hydrogel or ointment group (22 in each group) and instructed to apply the product twice daily for 4 weeks. Subjects were stratified according to ethnicity, age (adults versus minors), and disease severity and extent and assessed at baseline, week 2, and week 4. Seven parameters were utilized at each assessment: EASI, BSA, Atopic Dermatitis Severity Index (ADSI), target lesion assessment, subjective irritation symptoms, corneometry (with a Corneometer CM 825 [Courage + Khazaka, Köln, Germany]) to measure the target lesion’s moisture content in the stratum corneum), and TEWL (with a DermaLab [Cortex Technology, Hadsund, Denmark]). The subjects also answered a vehicle preference questionnaire at week 2 and week 4. Both hydrogel and ointment groups demonstrated decrease in EASI at week 2 and week 4 (p < 0.05) compared to baseline, with overall decreases similar between groups. Decrease in BSA was observed in both groups but was only significant for the ointment group at week 4 (p < 0.05), with no significant difference between the groups. Both groups exhibited significant decrease in ADSI, severity of target lesion, and subjective irritation symptoms at week 2 and week 4 compared to baseline (p < 0.05 for all three parameters). Corneometry did not reveal a significant change in either group at week 2 or week 4. By week 4, both hydrogel and ointment groups exhibited a decrease in TEWL (p < 0.05). Patient preference revealed significant inclination for the desonide hydrogel over ointment (p < 0.05) on absorption at week 2, lack of greasiness at week 2, and absorption at week 4. No adverse events were encountered, and five subjects deviated from protocol, although trial efficacy was not affected. The hydrogel group consumed an average of 37.82 g of product, whereas the ointment group consumed 23.59 g. As the results indicate, desonide hydrogel 0.05 % and desonide ointment 0.05 % are comparable in resolving symptoms of mild-to-moderate atopic dermatitis, and thus this study allowed for observation of differences attributable to the vehicle [38•]. No changes were observed in skin hydration as measured by corneometry in either group, which is contrary to findings from previous studies that demonstrated hydrogel’s significant moisturizing effects [39].
A pilot study published in February 2012 also investigated TEWL (measured by corneometry) in 20 subjects with mild-to-moderate atopic dermatitis treated with a hydrogel vehicle, which resulted in a statistically significant enhancement of skin hydration when compared with a moisturizing lotion control (Eucerin Lotion, Beiersdorf, Hamburg, Germany) [40]. The patient preference of hydrogel compared with ointment for absorbability and lack of greasiness may indicate better adherence to therapy, as the authors suggest. This study was limited by the fact that adherence was not separately evaluated, although the authors propose that the increased average use of hydrogel (37.82 g) compared to ointment (23.59 g) could indicate better compliance [41].
Pacha et al. [42•] reviewed the safety, efficacy, and patient acceptability of ceramide hyaluronic acid emollient foam in treating atopic dermatitis. Topical ceramides are superior to topical steroids or calcineurin inhibitors in terms of safety, as they do not carry the risks of skin atrophy, telangiectasia, or acne development; the potential for systemic absorption that accompanies topical steroids; or the theoretical risks that accompany topical calcineurin inhibitors. Safety of ceramides may differ based on the method obtained: synthetically or naturally. Natural ceramides may instinctively be the preferred agent to the general population and according to popular media influences, but these ingredients pose some disadvantages. In addition to being more expensive and more susceptible to contamination or infection than synthetic ceramides, a natural ceramide is capable of inhibiting keratinocyte proliferation and disrupting the action potential of mitochondria [42•]. The source of natural ceramides is bovine central nervous system and may cause of contamination [43].
Unlike natural ceramides, synthetic ceramides can be reduced to a more pure form, are less expensive, and—because they cannot cross the cell membrane [44]—are incapable of causing unwanted biologic effects. In cultured human keratinocytes, synthetic chemical mimics of ceramide did not produce cell toxicity, inhibit keratinocyte growth, or decrease mitochondrial membrane potential, whereas both exogenous cell-permeant and natural ceramides did [3].
In terms of efficacy, consistent use of topical ceramide emollients have been shown to decrease TEWL and improve desquamation [45]. Compared to calcineurin inhibitors and similar to pimecrolimus, ceramide emollient foam may produce comparable symptomatic resolve. The well-known potential adverse side effects of topical corticosteroids may be avoided by substituting a ceramide emollient foam for long-term, highly safe maintenance therapy [39].
The novel ingredients that are present in these emollients are also present in OTC emollients. The designation of a 510(k) device is conferred by the FDA. This simply means that they must be shown to be similar to previously cleared devices and then are available by prescription. They are FDA cleared, not FDA approved. Overwhelmingly, the ingredients available in these devices are also available in OTC products [46]. However, these products do appear in more clinical trials and are vetted by some of the rigor of scientific examination that OTC products are not.
Conclusions
Numerous advancements have taken place in recent years to further the understanding of the pathophysiology behind atopic dermatitis. With a prevalence as high as 20 % in industrialized countries, understanding these mechanisms of manifestation is pivotal to developing new, targeted therapy. Decreased genetic expression of filaggrin has been associated with peanut allergy, whereas size variation of filaggrin has been associated with a risk of eczema. Ultimately, filaggrin appears to result in a disorganized cytoskeleton matrix. An optimal skin barrier is maintained, in part, by filaggrin and profilaggrin. A mutation in the filaggrin gene sequence may not be sufficient to account for the various allergies related to its presence, including allergic sensitization, eczema-associated asthma, allergic rhinitis, and peanut allergy. Instead, mutations of filaggrin resulting in loss of function may predispose individuals to these conditions.
Recently, IL-31 has been described as a cytokine present in higher concentrations of atopic skin. This relationship introduces a future target for additional research, as IL-31 was correlated to scratching counts in experimental models with mice, but not in biopsies of pruritic human skin. The exact relationship between atopic human skin and IL-31 has yet to be described but holds promise for the future understanding of pathophysiology of eczema. Additionally, IL-31 may become a target for therapy, similar to other immunomodulators currently on the market.
The management of eczema is directed at several mechanisms: suppress flares with immunosuppressants and immunomodulators, prevent flares with immunomodulators; and protect the skin barrier, and maintain skin integrity while taking measures to prevent secondary infection of the vulnerable barrier. Draelos [33•] demonstrated a patient preference—and, thus, potential for improved patient compliance—for emollient foam over emollient cream in mild-to-moderate atopic dermatitis and at least 10 % BSA involvement. Miller et al. [34] compared Atopiclair, EpiCeram, and Aquaphor in a study with 39 total subjects and concluded that the OTC moisturizer (Aquaphor) was significantly more cost-effective at controlling disease than the prescription devices (Atopiclair and EpiCeram) in patients with mild-to-moderate eczema and at least 1 % BSA involvement. However, this low BSA requirement could have limited the ability to generalize these results. Jensen et al. [37] compared the efficacy of topical pimecrolimus with topical bethamethsone in treating atopic skin and evaluated gene expression in each group. Although betamethasone had a greater effect on reducing expression of numerous cytokines and chemotactic factors (of which pimecrolimus reduces many but to a lesser degree or at a slower rate), betamethasone also reduced the expression of several keratin variants and structural factors (of which pimecrolimus does not reduce at all or does so to a lesser extent). These analyses indicate steroids have a role in controlling acute flares but compared to pimecrolimus may negatively affect the integrity of the skin if used long term. Trookman et al. [38•] compared desonide hydrogel with desonide ointment and found no difference in moisturization of the skin, as measured via corneometry. Patients, however, favored the hydrogel over the ointment, which could impact therapy adherence.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–94. PubMed PMID: 18385500.
• Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. doi:10.1038/jid.2011.393. Epub 2011 Dec 8. PubMed PMID: 22158554. This paper is an excellent, comprehensive discussion of the molecular details and clinical implications of filaggrin, and the consequences of its alterations. Insight is provided into where research began (direct correlation of loss-of-function mutation in filaggrin causes ichthyosis vulgaris) through what subsequent research has unveiled (size variation in filaggrin results in varying degrees of atopic dermatitis). This gene is a pivotal example of how genetic defects can result in atopic dermatitis and other disorders of keratinization, which have been long recognized and described yet poorly understood in terms of their origin or pathophysiology.
Uchida Y, Holleran WM, Elias PM. On the effects of topical synthetic pseudoceramides: comparison of possible keratinocyte toxicities provoked by the pseudoceramides, PC104 and BIO391, and natural ceramides. J Dermatol Sci. 2008;51(1):37–43. Epub 2008 Apr 8. PubMed PMID: 18396015; PubMed Central PMCID: PMC2410086.
Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–72. Review. PubMed PMID: 19043850.
Jungersted JM, Scheer H, Mempel M, et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911–8.
Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. doi:10.1038/jid.2011.393. Epub 2011 Dec 8.
Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. doi:10.1038/jid.2011.393. Epub 2011 Dec 8.
Presland RB, Haydock PV, Fleckman P, et al. Characterization of the human epidermal profilaggrin gene. Genomic organization and identificationof an S-100-like calcium binding domain at the amino terminus. J Biol Chem. 1992;267:23772–81.
Sandilands A, Sutherland C, Irvine A, et al. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–94.
Manabe M, Sanchez M, Sun TT, et al. Interaction of filaggrin with keratin filaments during advanced stages of normal human epidermal differentiation and in ichthyosis vulgaris. Differentiation. 1991;48:43–50.
Gruber R, Elias PM, Crumrine D, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–63.
Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40.
Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43–8.
Kezic S, Kemperman PM, Koster ES, et al. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128:2117–9.
Miajlovic H, Fallon PP, Irvine AD, et al. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–90.
Fluhr JW, Elias PM, Man MQ, et al. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J Invest Dermatol. 2010;130:2141–4.
Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. doi:10.1038/jid.2011.393. Epub 2011 Dec 8.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-offunction variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6.
van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433.
Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121(872–7):e879.
Schuttelaar ML, Kerkhof M, Jonkman MF, et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy. 2009;64:1758–65.
• Brown SJ, Asai Y, Cordell HJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–7. This paper demonstrates and describes the most recent evidence regarding another manifestation of a loss-of-function mutation in filaggrin: peanut allergy. Understanding this mechanism behind this relationship could provide tremendous insight into the role of filaggrin in allergies and atopy in general.
Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. doi:10.1038/jid.2011.393. Epub 2011 Dec 8.
De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3 Pt 2):949–63. doi:10.1038/jid.2011.435. Epub 2012 Jan 5. PubMed PMID: 22217737; PubMed Central PMCID: PMC3279586.
De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3 Pt 2):949–63. doi:10.1038/jid.2011.435. Epub 2012 Jan 5. PubMed PMID: 22217737; PubMed Central PMCID: PMC3279586.
Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60.
Takaoka A, Arai I, Sugimoto M, Honma Y, Futaki N, Nakamura A, Nakaike S. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp Dermatol. 2006;15(3):161–7.
• Baron JM, Lüscher B. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012;92(1):5–6. PubMed PMID: 22215012. This paper describes a novel relationship between IL-31 and atopic dermatitis. This could unveil mechanisms of pathophysiology and potential therapy directed at eczema. More research is required to determine if IL-31 is related to pruritus, atopy, or other specific manifestations.
Cox H, Lloyd K, Williams H, Arkwright PD, Brown T, Clark C, Campbell M, Gore C, Hardman C, Langford A, Lewis-Jones S, Lawton S, Ridd M, Russell L, Sohi D, Turnbull R, Venter C, Warner JO, Science and Research Department, Royal College of Paediatrics and Child Health. Emollients, education and quality of life: the RCPCH care pathway for children with eczema. Arch Dis Child. 2011;96 Suppl 2:i19–24. PubMed PMID: 22053062.
Shams K, Grindlay DJ, Williams HC. What’s new in atopic eczema? An analysis of systematic reviews published in 2009–2010. Clin Exp Dermatol. 2011;36(6):573–7. doi:10.1111/j.1365-2230.2011.04078. x quiz 577-8. Epub 2011 Jul 1. Review. PubMed PMID: 21718344.
Green JS, Andersen K, Wilkin J, Siegfried E. Understanding over-the-counter medications and prescription devices for eczema: potential consequences for consumers. J Am Acad Dermatol. 2010;63(6):1094–8.
Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164(2):415–28. doi:10.1111/j.1365-2133.2010.10030.x. Epub 2010 Nov 23. Review.
• Draelos ZD. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J Cosmet Dermatol. 2011;10(3):185–8. doi:10.1111/j.1473-2165.2011.00568.x. PubMed PMID: 21896129. This study was valuable in assessing the notion of cost-effectiveness in treating atopic dermatitis, which is important to address because patients who do not perceive that therapy worth the expense will be less likely to adhere to the plan than those who do. In mild-to-moderate disease, OTC ceramide-containing creams may be superior and valid alternatives to prescription devices like Atopoclair and Epiceram when considering cost-effectiveness. This conclusion is particularly relevant for maintenance therapy but less so in the management of preventing or suppressing flares of atopic dermatitis.
Miller DW, Koch SB, Yentzer BA, Clark AR, O’Neill JR, Fountain J, Weber TM, Fleischer Jr AB. An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol. 2011;10(5):531–7.
Steele T, Pawaskar M, Balkrishnan R, Fleischer A, Feldman SR. Does cost-effectiveness play a role in clinical trials? Dermatol Ther. 2007;20(2):110–9.
• Jensen JM, Scherer A, Wanke C, Bräutigam M, Bongiovanni S, Letzkus M, Staedtler F, Kehren J, Zuehlsdorf M, Schwarz T, Weichenthal M, Fölster-Holst R, Proksch E. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy. 2012;67(3):413–23. doi:10.1111/j.1398-9995.2011.02747.x. Epub 2011 Dec 6. PubMed PMID: 22142306. This paper describes the various, specific genes whose expression is affected by betamethasone and pimecrolimus. The conclusions give statistically significant support to the notion that topical steroid use impairs the integrity of the skin as compared to pimecrolimus. The authors also describe various cytokines and chemotactic agents of which the two therapies influence concentrations and expression in treated skin.
Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, Schwarz T, Fölster-Holst R, Proksch E. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;123(5):1124–33. Erratum in: J Allergy Clin Immunol. 2009 Nov;124(5):1038. PubMed PMID: 19410693.
• Trookman NS, Rizer RL. Randomized controlled trial of desonlde hydrogel 0.05 % versus desonide ointment 0.05 % in the treatment of mild-to-moderate atopic dermatitis. J Clin Aesthet Dermatol. 2011;4(11):34–8. PubMed PMID: 22125657; PubMed Central PMCID: PMC3225140. This paper contradicts previous studies by concluding that hydrogel desonide is not superior in minimizing TEWL as compared to ointment desonide. This argues against recommending hydrogel over the ointment unless compliance may be improved with the hydrogel.
Trookman NS, Rizer RL, Ford RO, Gotz V. The stratum corneum and atopic dermatitis: moisturizing advantages of a novel desonide hydrogel treatment. Presented at: 66th Annual Meeting of the American Academy of Dermatology; February 1–5, 2008; San Antonio, TX.
Kircik LH. Transepidermal water loss (TEWL) and corneometry with hydrogel vehicle in the treatment of atopic dermatitis: a randomized, investigator-blind pilot study. J Drugs Dermatol. 2012;11(2):180–4.
Trookman NS, Rizer RL. Randomized controlled trial of desonide hydrogel 0.05 % versus desonide ointment 0.05 % in the treatment of mild-to-moderate atopic dermatitis. J Clin Aesthet Dermatol. 2011;4(11):34–8. PubMed PMID: 22125657; PubMed Central PMCID: PMC3225140.
• Pacha O, Hebert AA. Treating atopic dermatitis: safety, efficacy, and patient acceptability of a ceramide hyaluronic acid emollient foam. Clinical, Cosmetic and Investigational Dermatology, In press. This review compares the safety of and efficacy of barrier protection therapies in atopic dermatitis. The controversy regarding the synthetic versus natural ceramides contained in these products is also addressed, as are several aspects to consider when choosing a topical product for patients with eczema.
Adkin A, Webster V, Arnold ME, Wells GAH, Matthews D. Estimating the impact on the food chain of changing bovine spongiform encephalopathy (BSE) control measures: The BSE Control Model, Preventive Veterinary Medicine, Volume 93, Issues 2-3, 1 February 2010, Pages 170–182, ISSN 0167-5877, doi:10.1016/j.prevetmed.2009.09.018.
Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Journal of Biological Chemistry. 1997;272:11369.
Kikuchi K, Tagami H, the Japanese Cosmetic Scientist Task Force for Skin Care of Atopic Dermatitis. Noninvasive biophysical assessments of the efficacy of a moisturizing cosmetic cream base for patients with atopic dermatitis during different seasons. Br J Dermatol. 2008;158:969–78.
Draelos ZD. New treatments for restoring impaired epidermal barrier permeability: skin barrier repair creams. linics in Dermatology. 2012;30:345–8.
Disclosure
O. Pacha: none; B. L. Sambrano: none; A. A. Hebert: consulting fee/honorarium from Onstat, Novartis, Astellas; grants from Novartis, Astellas, Promius; and payment for lectures from Novartis, Astellas, Promius, Onstat.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacha, O., Sambrano, B.L. & Hebert, A.A. Skin Barrier Repair in Eczema: A Review of Current Understanding of Pathophysiology and Treatment. Curr Derm Rep 1, 115–122 (2012). https://doi.org/10.1007/s13671-012-0018-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-012-0018-6