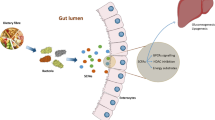
Abstract
Purpose of Review
This narrative review will discuss how the intake of specific protein sources (animal and vegetable) providing specific amino acids can modulate the gut microbiota composition and generate toxins. A better understanding of these interactions could lead to more appropriate dietary recommendations to improve gut health and mitigate the risk of complications promoted by the toxic metabolites formed by the gut microbiota.
Recent Findings
Gut microbiota is vital in maintaining human health by influencing immune function and key metabolic pathways. Under unfavorable conditions, the gut microbiota can produce excess toxins, which contribute to inflammation and the breakdown of the integrity of the intestinal barrier. Genetic and environmental factors influence gut microbiota diversity, with diet playing a crucial role. Emerging evidence indicates that the gut microbiota significantly metabolizes amino acids from dietary proteins, producing various metabolites with beneficial and harmful effects.
Summary
Amino acids such as choline, betaine, l-carnitine, tyrosine, phenylalanine, and tryptophan can increase the production of uremic toxins when metabolized by intestinal bacteria. The type of food source that provides these amino acids affects the production of toxins. Plant-based diets and dietary fiber are associated with lower toxin formation than animal-based diets due to the high amino acid precursors in animal proteins.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, Cammarota G. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20(22):4742–9.
Mafra D, Kalantar-Zadeh K, Moore LW. New tricks for old friends: treating gut microbiota of patients with CKD. J Ren Nutr. 2021;31(5):433–7. https://doi.org/10.1053/j.jrn.2021.07.002.
Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, Stenvinkel P. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients. 2019;11(3):496. https://doi.org/10.3390/nu11030496.
Coutinho-Wolino KS, de F Cardozo LFM, de Oliveira Leal V, Mafra D, Stockler-Pinto MB. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far. Eur J Nutr. 2021;60(7):3567–84. https://doi.org/10.1007/s00394-021-02491-6.
Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. https://doi.org/10.1038/s41575-018-0061-2.
Massy ZA, Drueke TB. Diet-microbiota interaction and kidney disease progression. Kidney Int. 2021;99(4):797–800. https://doi.org/10.1016/j.kint.2020.11.006.
Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci. 2019;20(2):145–54. https://doi.org/10.2174/1389203719666180514145437.
Ma J, Li Z, Zhang W, et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020;10(1):15792.
Meng C, Feng S, Hao Z, Dong C, Liu H. Changes in gut microbiota composition with age and correlations with gut inflammation in rats. PLoS ONE. 2022;17(3): e0265430.
Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. https://doi.org/10.3390/microorganisms7010014.
Esgalhado M, Kemp JA, Damasceno NR, Fouque D, Mafra D. Short-chain fatty acids: a link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017;12:1413–25. https://doi.org/10.2217/fmb-2017-0059.
• Ferreira RDS, Mendonça LABM, Ribeiro CFA, et al. Relationship between intestinal microbiota, diet and biological systems: an integrated view. Crit Rev Food Sci Nutr. 2022;62(5):1166–86. https://doi.org/10.1080/10408398.2020.1836605. Addresses the complex interaction between the intestinal microbiota, diet, and biological systems of the human body. It highlights how the composition of the intestinal microbiota can be influenced by diet and how these changes can affect different aspects of health.
Martinez JE, Kahana DD, Ghuman S, et al. Unhealthy lifestyle and gut dysbiosis: a better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). 2021;12: 667066. https://doi.org/10.3389/fendo.2021.667066.
DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22(5):1137–50. https://doi.org/10.1097/MIB.0000000000000750.
Inoue R, Ohue-Kitano R, Tsukahara T, et al. Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr. 2017;61(3):217–21. https://doi.org/10.3164/jcbn.17-44.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. https://doi.org/10.1038/nature11450.
Lekawanvijit S, Kompa AR, Krum H. Protein-bound uremic toxins: a long overlooked culprit in cardiorenal syndrome. Am J Physiol Renal Physiol. 2016;311(1):F52-62.
Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63(5):1934–43.
Chen YY, Chen DQ, Chen L, Liu JR, Vaziri ND, Guo Y, et al. Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J Transl Med. 2019;17(1).
Popkov VA, Silachev DN, Zalevsky AO, Zorov DB, Plotnikov EY. Mitochondria as a source and a target for uremic toxins. Int J Mol Sci. 2019;20(12):3094.
Valkenburg S, Glorieux G, Vanholder R. Uremic toxins and cardiovascular system. Cardiol Clin. 2021;39(3):307–18.
••Harlacher E, Wollenhaupt J, Baaten CCFMJ, Noels H. Impact of uremic toxins on endothelial dysfunction in chronic kidney disease: a systematic review. Int J Mol Sci. 2022;23(1):531 Endothelial dysfunction is a common issue in CKD patients and is associated with serious cardiovascular complications. Studying these toxins and their effect on the endothelium can provide crucial insights into the mechanisms underlying cardiovascular complications in CKD.
Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5(6):1302–8.
Pieniazek A, Bernasinska-Slomczewska J, Gwozdzinski L. Uremic toxins and their relation with oxidative stress induced in patients with CKD. Int J Mol Sci. 2021;22(12):6196.
Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84(4):733–44.
Addi T, Poitevin S, McKay N, et al. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-kappa B signaling pathway in human endothelial cells. Arch Toxicol. 2019;93(1):121–36. https://doi.org/10.1007/s00204-018-2328-3.
Opdebeeck B, Maudsley S, Azmi A, De Maré A, De Leger W, Meijers B, et al. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol. 2019;30(5):751–66.
Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2012;24(1):88–99.
Fujii H, Goto S, Fukagawa M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins. 2018;10(5):202.
Cohen G. Immune dysfunction in uremia 2020. Toxins. 2020;12(7):439.
Tecklenborg J, Clayton D, Siebert S, Coley SM. The role of the immune system in kidney disease. Clin Exp Immunol. 2018;192(2):142–50.
Sun Y, Johnson C, Zhou J, et al. Uremic toxins are conditional danger- or homeostasis-associated molecular patterns. Front Biosci (Landmark Ed). 2018;23(2):348–87.
Azevedo MLV, Bonan NB, Dias G, Brehm F, Steiner TM, Souza WM, et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicology Letters [Internet]. 2016;263:1–5.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1).
Noce A, Marrone G, Di Daniele F, Ottaviani E, Wilson Jones G, Bernini R, et al. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients. 2019;11(5):1073.
Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A. Influence of Mediterranean diet on human gut microbiota. Nutrients. 2021;13(1):7. https://www.mdpi.com/2072-6643/13/1/7.
Wang L, Du M, Wang K, Khandpur N, Rossato SL, Drouin-Chartier JP, et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ [Internet]. 2022;378: e068921.
••Lauriola M, Farré R, Evenepoel P, Overbeek SA, Meijers B. Food-derived uremic toxins in chronic kidney disease. Toxins. 2023;15(2) The article highlights the importance of diet in the management of CKD and how specific food choices can influence the prognosis and progression of the diseae.
Cao Y, Liu S, Liu K, Abbasi IHR, Cai C, Yao J. Molecular mechanisms relating to amino acid regulation of protein synthesis. Nutr Res Rev. 2019;32(2):183–91. https://doi.org/10.1017/S0954422419000052.
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44(4):1107–13. https://doi.org/10.1007/s00726-012-1444-2.
Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. https://doi.org/10.1007/s00394-017-1445-8.
USDA, Agricultural Research Service. National Nutrient Database for Standard Reference. Washington (DC): USDA; 2013 [cited 2022 Sep 27]. https://fdc.nal.usda.gov/ .
Leermakers ET, Moreira EM, Kiefte-de Jong JC, et al. Effects of choline on health across the life course: a systematic review. Nutr Rev. 2015;73(8):500–22. https://doi.org/10.1093/nutrit/nuv010.
Lewis ED, Subhan FB, Bell RC, et al. Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr. 2014;112(1):112–21. https://doi.org/10.1017/S0007114514000555.
Zhu Y, Li Q, Jiang H. Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. APMIS. 2020;128(5):353–66. https://doi.org/10.1111/apm.13038.
Glorieux G, Nigam SK, Vanholder R, Verbeke F. Role of the microbiome in gut-heart-kidney cross talk. Circ Res. 2023;132(8):1064–83. https://doi.org/10.1161/CIRCRESAHA.123.321763.
Thomas MS, Huang L, Garcia C, Sakaki JR, Blesso CN, Chun OK, Fernandez ML. The effects of eggs in a plant-based diet on oxidative stress and inflammation in metabolic syndrome. Nutrients. 2022;14(12):2548. https://doi.org/10.3390/nu14122548.
• Alvarenga L, Ferreira MS, Kemp JA, Mafra D. The role of betaine in patients with chronic kidney disease: a narrative review. Curr Nutr Rep. 2022;11(3):395–406. https://doi.org/10.1007/s13668-022-00426-z. Understanding the role of betaine in CKD may lead to new therapeutic approaches or dietary recommendations to improve quality of life and clinical outcomes in these patients.
Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC Natl Acad Press. Washington, D.C.: National Academies Press. 1998.
Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Rath S, Rud T, Pieper DH, Vital M. Potential TMA-producing bacteria are ubiquitously found in Mammalia. Front Microbiol. 2020;10:2966. https://doi.org/10.3389/fmicb.2019.02966.
Manor O, Zubair N, Conomos MP, et al. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24(4):935–46. https://doi.org/10.1016/j.celrep.2018.06.096.
Yoo W, Zieba JK, Foegeding NJ, et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. 2021;373(6556):813–8. https://doi.org/10.1126/science.aba3683.
Zhu C, Sawrey-Kubicek L, Beals E, et al. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: a pilot study. Nutr Res. 2020;77:62–72. https://doi.org/10.1016/j.nutres.2020.03.005.
Rajakovich LJ, Fu B, Bollenbach M, Balskus EP. Elucidation of an anaerobic pathway for metabolism of l-carnitine-derived γ-butyrobetaine to trimethylamine in human gut bacteria. Proc Natl Acad Sci U S A. 2021;118(32): e2101498118. https://doi.org/10.1073/pnas.2101498118.
Dai L, Massy ZA, Stenvinkel P, et al. The association between TMAO, CMPF, and clinical outcomes in advanced chronic kidney disease: results from the European QUALity (EQUAL) Study. Am J Clin Nutr. 2022;116(6):1842–51. https://doi.org/10.1093/ajcn/nqac278.
Mafra D, Kemp JA, Leal VO, et al. Consumption of fish in chronic kidney disease - a matter of depth. Mol Nutr Food Res. 2023;67(9): e2200859. https://doi.org/10.1002/mnfr.202200859.
Mu Y, Bian C, Liu R, et al. Whole genome sequencing of a snailfish from the Yap Trench (~7,000 m) clarifies the molecular mechanisms underlying adaptation to the deep sea. PLoS Genet. 2021;17(5): e1009530. https://doi.org/10.1371/journal.pgen.1009530.
Huang M, Wei R, Wang Y, Su T, Li P, Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol. 2018;16:303–13.
Hase A, Jung SE, aan het Rot M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol Biochem Behav. 2015;133:1–6. https://doi.org/10.1016/j.pbb.2015.03.008.
Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. 2013;65(4):341–9. https://doi.org/10.1002/iub.1150.
Young VR, Borgonha S. Nitrogen and amino acid requirements: the Massachusetts Institute of Technology amino acid requirement pattern. J Nlltr. 2000;130:1841S-49S.
Bhargava S, Merckelbach E, Noels H, Vohra A, Jankowski J. Homeostasis in the gut microbiota in chronic kidney disease. Toxins (Basel). 2022;14(10):648. https://doi.org/10.3390/toxins14100648.
Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-Cresyl sulfate. Toxins (Basel). 2017;9(2):52. https://doi.org/10.3390/toxins9020052.
Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57:523–35.
Jiao M, He W, Ouyang Z, Shi Q, Wen Y. Progress in structural and functional study of the bacterial phenylacetic acid catabolic pathway, its role in pathogenicity and antibiotic resistance. Front Microbiol. 2022;13: 964019. https://doi.org/10.3389/fmicb.2022.964019.
Teufel R, Mascaraque V, Ismail W, et al. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci U S A. 2010;107(32):14390–5. https://doi.org/10.1073/pnas.1005399107.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–24. https://doi.org/10.1016/j.chom.2018.05.003.
Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. 2021;22:6–2973. https://doi.org/10.3390/ijms22062973.
Zhang ZW, Gao CS, Zhang H, et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm Sin B. 2022;12(8):3298–312. https://doi.org/10.1016/j.apsb.2022.02.032.
Gibson EL. Tryptophan supplementation and serotonin function: genetic variations in behavioural effects. Proc Nutr Soc. 2018;77(2):174–88. https://doi.org/10.1017/S0029665117004451.
Matsumoto T, et al. Role of S-equol, indoxyl sulfate, and trimethylamine N-oxide on vascular function. Am J Hypertens. 2020;33(9):793.
Leong SC, Sirich TL. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins (Basel). 2016;8(12):358.
Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos. 2015;43:1522–35.
Gryp T, Huys GRB, Joossens M, Van Biesen W, Glorieux G, Vaneechoutte M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int J Mol Sci. 2020;21(6):1986. https://doi.org/10.3390/ijms21061986.
Gorissen SHM, Trommelen J, Kouw IWK, Holwerda AM, Pennings B, Groen BBL, et al. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr 2020:nxaa024.
Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47.
Derrien M, Veiga P. Rethinking diet to aid human-microbe symbiosis. Trends Microbiol. 2017;25:100–12.
Fernandes ALF, Borges NA, Black AP, Anjos JD, Silva GSD, Nakao LS, Mafra D. Dietary intake of tyrosine and phenylalanine, and p-cresyl sulfate plasma levels in non-dialyzed patients with chronic kidney disease. J Bras Nefrol. 2020;42(3):307–14.
Koppe L, Fouque D, Soulage CO. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins (Basel). 2018;10(4):155.
Cases A, Cigarrán-Guldrís S, Mas S, Gonzalez-Parra E. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients. 2019;11(6):1263.
Mocanu CA, Simionescu TP, Mocanu AE, Garneata L. Plant-based versus animal-based low protein diets in the management of chronic kidney disease. Nutrients. 2021;13(11):3721.
Mariotti F, Gardner CD. Dietary protein and amino acids in vegetarian diets-a review. Nutrients. 2019;11(11):2661.
Ikizler TA, Cuppari L. The 2020 updated KDOQI clinical practice guidelines for nutrition in chronic kidney disease. Blood Purif. 2021;50(4–5):667–71.
Moore LW, Byham-Gray LD, Scott Parrott J, Rigassio-Radler D, Mandayam S, Jones SL, Mitch WE, Osama GA. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013;83(4):724–32.
Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2017;20(1):77–85.
Black AP, Anjos JS, Cardozo L, Carmo FL, Dolenga CJ, Nakao LS, de Carvalho FD, Rosado A, Carraro Eduardo JC, Mafra D. Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J Ren Nutr. 2018;28(3):208–14.
Losasso C, Eckert EM, Mastrorilli E, Villiger J, Mancin M, Patuzzi I, et al. Assessing the influence of vegan, vegetarian and omnivore oriented Westernized dietary styles on human gut microbiota: a cross sectional study. Front Microbiol. 2018;9:317.
Jain A, Li XH, Chen WN. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express. 2018;8:104.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 2015;22:971–82.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Sheflin AM, Melby CL, Carbonero F, Weir TL. Linking dietary patterns with gut microbial composition and function. Gut Microbes. 2016;8:113–29.
Salmean YA, Segal MS, Langkamp-Henken B, Canales MT, Zello GA, Dahl WJ. Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr. 2013;23:e29–32.
Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185.
Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184–96.
Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Contributions
Livia Alvarenga, together with the other authors Julie A. Kemp, Beatriz G. Baptista, Marcia Ribeiro, Ligia Soares Lima and Denise Mafra, wrote the main text of the manuscript. The tables were created by Livia Alvarenga and figures 1 and 3 by Denise Mafra. Figure 2 was created by author Marcia Ribeiro. All authors carried out an intense bibliographical search to produce this manuscript. Furthermore, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvarenga, L., Kemp, J.A., Baptista, B.G. et al. Production of Toxins by the Gut Microbiota: The Role of Dietary Protein. Curr Nutr Rep (2024). https://doi.org/10.1007/s13668-024-00535-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s13668-024-00535-x