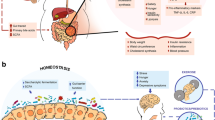
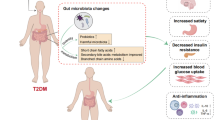
Abstract
Purpose of Review
This review delves into the complex interplay between obesity-induced gut microbiota dysbiosis and the progression of type 2 diabetes mellitus (T2DM), highlighting the potential of natural products in mitigating these effects. By integrating recent epidemiological data, we aim to provide a nuanced understanding of how obesity exacerbates T2DM through gut flora alterations.
Recent Findings
Advances in research have underscored the significance of bioactive ingredients in natural foods, capable of restoring gut microbiota balance, thus offering a promising approach to manage diabetes in the context of obesity. These findings build upon the traditional use of medicinal plants in diabetes treatment, suggesting a deeper exploration of their mechanisms of action.
Summary
This comprehensive manuscript underscores the critical role of targeting gut microbiota dysbiosis in obesity-related T2DM management and by bridging traditional knowledge with current scientific evidence; we highlighted the need for continued research into natural products as a complementary strategy for comprehensive diabetes care.

Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- ATF2:
-
Activating transcription factor 2
- AMPK/PI3K/Akt/GSK3β:
-
AMP-activated protein kinase/phosphatidylinositol 3-kinase/serine/threonine protein kinase b/glycogen synthase kinase-3 beta
- CAT:
-
Catalase
- DM:
-
Diabetes mellitus
- CRP:
-
C-reactive protein
- FGF2:
-
Fibroblast growth factor 2
- FOXO1:
-
Forkhead box protein O1
- GSK-3β:
-
Glycogen synthase kinase 3 beta
- GPx:
-
Glutathione peroxidase
- GLP-1:
-
Glucagon-like peptide 1
- GLUT4:
-
Glucose transporter type 4
- GSH:
-
Glutathione
- H2O2:
-
Hydrogen peroxide
- IL-6:
-
Interleukin 6
- IRS1:
-
Insulin receptor substrate 1
- ICAM1:
-
Intercellular adhesion molecule 1
- JNK:
-
Jun N-terminal kinase
- MCP-1:
-
Monocyte chemoattractant protein-1
- mRNA:
-
Messenger RNA
- Muc2:
-
Mucin 2
- mTOR:
-
Mammalian target of rapamycin
- NO:
-
Nitrous oxide
- NF-κB:
-
Nuclear factor kappa B
- NRK 52E:
-
Rat renal proximal tubular epithelial cells
- PPARG:
-
Peroxisome proliferator-activated receptor gamma
- PDX1:
-
Pancreatic and duodenal homeobox 1
- p38MAPK:
-
P38 Mitogen-activated protein kinases
- ROS:
-
Reactive oxygen species
- Rab27a/Slp4:
-
Ras-related protein/synaptotagmin-like protein 4
- SOD:
-
Superoxide dismutase
- SIRT1:
-
Sirtuin 1
- SLC2A4:
-
Solute carrier family 2 member 4
- TNFα:
-
Tumor necrosis factor-alpha
- TGF-β1:
-
Transforming growth factor beta 1
- TFF3:
-
Trefoil factor family 3
- T2DM:
-
Type 2 diabetes mellitus
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. https://doi.org/10.1016/S0140-6736(23)01301-6. The study systematically analyzes the global diabetes burden from 1990 to 2021 and projects an increasing prevalence up to 2050, emphasizing the urgent need for comprehensive public health strategies.
• Saeedi P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. The study explores the multifaceted health benefits of Quercetin, highlighting its therapeutic potential across various medical conditions due to its antioxidant and anti-inflammatory properties.
Sasako T, Yamauchi T, Ueki K. Intensified multifactorial intervention in patients with type 2 diabetes mellitus. Diabetes Metab J. 2023;47(2):185–97.
ElSayed NA, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–40.
Dludla PV, et al. Pancreatic β-cell dysfunction in type 2 diabetes: implications of inflammation and oxidative stress. World J Diabetes. 2023;14(3):130–46.
Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne). 2021;12:706978.
Mutalub YB, et al. Gut microbiota modulation as a novel therapeutic strategy in cardiometabolic diseases. Foods. 2022;11(17):2575.
Hou K, et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7(1):135.
Álvarez J, et al. Gut microbes and health. Gastroenterología y Hepatología (English Edition). 2021;44(7):519–35.
Bajinka O, et al. The gut microbiota pathway mechanisms of diabetes. AMB Express. 2023;13(1):16.
Ayakdaş G, Ağagündüz D. Microbiota-accessible carbohydrates (MACs) as novel gut microbiome modulators in noncommunicable diseases. Heliyon. 2023;9(9):e19888.
Mazhar M, Zhu Y, Qin L. The interplay of dietary fibers and intestinal microbiota affects type 2 diabetes by generating short-chain fatty acids. Foods. 2023;12(5):1023.
Wu J, Yang K, Fan H, Wei M, Xiong Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1114424. https://doi.org/10.3389/fendo.2023.1114424.
Fakharian F, Thirugnanam S, Welsh DA, et al. The role of gut dysbiosis in the loss of intestinal immune cell functions and viral pathogenesis. Microorganisms. 2023;11(7):1849. https://doi.org/10.3390/microorganisms11071849.
Kang GG, et al. Diet-induced gut dysbiosis and inflammation: key drivers of obesity-driven NASH. iScience. 2023;26(1):105905.
Basak S, et al. Dietary fats and the gut microbiota: their impacts on lipid-induced metabolic syndrome. J Funct Foods. 2022;91:105026.
Jia L, et al. Pharmacomicrobiomics and type 2 diabetes mellitus: a novel perspective towards possible treatment. Front Endocrinol (Lausanne). 2023;14:1149256.
Tang X, et al. Angelica polysaccharides relieve blood glucose levels in diabetic KKAy mice possibly by modulating gut microbiota: an integrated gut microbiota and metabolism analysis. BMC Microbiol. 2023;23(1):281.
Li R, et al. Bi-directional interactions between glucose-lowering medications and gut microbiome in patients with type 2 diabetes mellitus: a systematic review. Genes. 2023;14(8):1572.
Sorrenti V, Burò I, Consoli V, Vanella L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: biochemical aspects. Int J Mol Sci. 2023;24(3):2019. https://doi.org/10.3390/ijms24032019.
• Patel BK, Patel KH, Moochhala SM. Gut microbiota intervention strategies using active components from medicinal herbs to evaluate clinical efficacy of type 2 diabetes – a review. Clin Transl Discov. 2023;3(1):e170. The study delves into the potential of activecompounds from medicinal herbs in modulating gut microbiota for Type 2 Diabetestreatment, underscoring the clinical significance of herbal interventions.
Yang F, Gao R, Luo X, Liu R, Xiong D. Berberine influences multiple diseases by modifying gut microbiota. Front Nutr. 2023;10:1187718. https://doi.org/10.3389/fnut.2023.1187718.
Cheng H, et al. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J Pharm Anal. 2022;12(4):541–55.
World Flora Online. https://www.worldfloraonline.org.
Puljiz Z, Kumric M, Vrdoljak J, et al. Obesity, gut microbiota, and metabolome: from pathophysiology to nutritional interventions. Nutrients. 2023;15(10):2236. https://doi.org/10.3390/nu15102236.
Duncan SH, et al. Links between diet, intestinal anaerobes, microbial metabolites and health. Biomedicines. 2023;11(5):1338.
Puljiz Z, Kumric M, Vrdoljak J, Martinovic D, Ticinovic Kurir T, Krnic MO, Urlic H, Puljiz Z, Zucko J, Dumanic P, Mikolasevic I, Bozic J. Obesity, gut microbiota, and metabolome: from pathophysiology to nutritional interventions. Nutrients. 2023;15(10):2236. https://doi.org/10.3390/nu15102236.
Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91.
Zhang D, et al. Short-chain fatty acids in diseases. Cell Commun Signal. 2023;21(1):212.
Lee JG, et al. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J Nutr Biochem. 2022;101:108926.
Blaak EE, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–55.
Lu Y, et al. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. 2016;6:37589.
Chandalia M, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342(19):1392–8.
Pingitore A, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19(2):257–65.
Liu S, Cheng L, Liu Y, Zhan S, Wu Z, Zhang X. Relationship between dietary polyphenols and gut microbiota: New clues to improve cognitive disorders, mood disorders and circadian rhythms. Foods. 2023;12(6):1309. https://doi.org/10.3390/foods12061309.
Bié J, et al. Polyphenols in health and disease: gut microbiota, bioaccessibility, and bioavailability. Compounds. 2023;3(1):40–72.
Zhang Q, Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:5003–14.
Liu L, et al. Gut microbiota: a new target for T2DM prevention and treatment. Front Endocrinol (Lausanne). 2022;13:958218.
Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022;12:834485. https://doi.org/10.3389/fcimb.2022.834485.
Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. Journal of Diabetes Investigation. 2018;9(1):5–12.
Freepik. https://www.freepik.com.
Jafari M, Juanson Arabit JG, Courville R, et al. The impact of Rhodiola rosea on biomarkers of diabetes, inflammation, and microbiota in a leptin receptor-knockout mouse model. Sci Rep. 2022;12(1):10581. https://doi.org/10.1038/s41598-022-14241-7.
Qi SS, et al. Salidroside from Rhodiola rosea L. attenuates diabetic nephropathy in STZ induced diabetic rats via anti-oxidative stress, anti-inflammation, and inhibiting TGF-β1/Smad pathway. J Funct Foods. 2021;77:104329.
Lee BH, Lee CC, Wu SC. Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J Sci Food Agric. 2014;94(11):2266–73.
Zhang C, et al. Extract of ice plant (Mesembryanthemum crystallinum) ameliorates hyperglycemia and modulates the gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. Food Funct. 2019;10(6):3252–61.
Gao Y, et al. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J Agric Food Chem. 2015;63(26):6019–26.
Pagliari S, Forcella M, Lonati E, et al. Antioxidant and anti-inflammatory effect of cinnamon (Cinnamomum verum J. Presl) bark extract after in vitro digestion simulation. Foods. 2023;12(3):452. https://doi.org/10.3390/foods12030452.
Jiang H, Cai M, Shen B, Wang Q, Zhang T, Zhou X. Synbiotics and gut microbiota: New perspectives in the treatment of type 2 diabetes mellitus. Foods. 2022;11(16):2438. https://doi.org/10.3390/foods11162438.
Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: A review. Nutrients. 2022;14(13):2773. https://doi.org/10.3390/nu14132773.
Ping H, Zhang G, Ren G. Antidiabetic effects of cinnamon oil in diabetic KK-Ay mice. Food Chem Toxicol. 2010;48(8–9):2344–9.
Van Hul M, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2018;314(4):E334-e352.
Gil-Sánchez I, et al. Supplementation with grape pomace in healthy women: changes in biochemical parameters, gut microbiota and related metabolic biomarkers. J Funct Foods. 2018;45:34–46.
Wang Y, et al. EVOO supplement prevents type 1 diabetes by modulating gut microbiota and serum metabolites in NOD mice. Life Sci. 2023;335:122274.
Winiarska-Mieczan A, Tomaszewska E, Donaldson J, Jachimowicz K. The role of nutritional factors in the modulation of the composition of the gut microbiota in people with autoimmune diabetes. Nutrients. 2022;14(12):2498. https://doi.org/10.3390/nu14122498.
Farràs M, et al. Modulation of the gut microbiota by olive oil phenolic compounds: implications for lipid metabolism, immune system, and obesity. Nutrients. 2020;12(8)
Martín-Peláez S, et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. Eur J Nutr. 2017;56(1):119–31.
Martín-Peláez S, et al. Influence of phenol-enriched olive oils on human intestinal immune function. Nutrients. 2016;8(4):213.
Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain RA. 6-gingerol, a bioactive compound of ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics. 2021;13(3):317. https://doi.org/10.3390/pharmaceutics13030317.
Samad MB, et al. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complement Altern Med. 2017;17(1):395.
Chen X, Pan S, Li F, Xu X, Xing H. Plant-derived bioactive compounds and potential health benefits: involvement of the gut microbiota and its metabolic activity. Biomolecules. 2022;12(12):1871. https://doi.org/10.3390/biom12121871.
Chen Z, Luo J, Jia M, Chai Y, Bao Y. Polygonatum sibiricum saponin Exerts Beneficial hypoglycemic effects in type 2 diabetes mice by improving hepatic insulin resistance and glycogen synthesis-related proteins. Nutrients. 2022;14(24):5222. https://doi.org/10.3390/nu14245222.
Xu J, Zhang J, Sang Y, et al. Polysaccharides from medicine and food homology materials: a review on their extraction, purification, structure, and biological activities. Molecules. 2022;27(10):3215. https://doi.org/10.3390/molecules27103215.
Chai Y, Luo J, Bao Y. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed Pharmacother. 2021;143:112155.
Ojo OA, et al. Gongronema latifolium Benth. leaf extract attenuates diabetes-induced neuropathy via inhibition of cognitive, oxidative stress and inflammatory response. J Sci Food Agric. 2020;100(12):4504–11.
Chen X, et al. Hypoglycemic mechanisms of Polygonatum sibiricum polysaccharide in db/db mice via regulation of glycolysis/gluconeogenesis pathway and alteration of gut microbiota. Heliyon. 2023;9(4):e15484.
Chukwudozie IK, Agbo MC, Ugwu KO, Ezeonu IM. Oral administration of Gongronema latifolium leaf extract modulates gut microflora and blood glucose of induced diabetic rats. J Pure Appl Microbiol. 2021;15(1):346–55.
Chukwudozie IK, et al. Oral Administration of Gongronema latifolium leaf extract modulates gut microflora and blood glucose of induced diabetic rats. J Pure Appl Microbiol. 2021;15:346+.
Shen X, et al. Polyphenol extracts from germinated mung beans can improve type 2 diabetes in mice by regulating intestinal microflora and inhibiting inflammation. Front Nutr. 2022;9:846409.
Li L, Tian Y, Zhang S, et al. Regulatory effect of mung bean peptide on prediabetic mice induced by high-fat diet. Front Nutr. 2022;9:913016. https://doi.org/10.3389/fnut.2022.913016.
Hou D, et al. A comparison between whole mung bean and decorticated mung bean: beneficial effects on the regulation of serum glucose and lipid disorders and the gut microbiota in high-fat diet and streptozotocin-induced prediabetic mice. Food Funct. 2020;11(6):5525–37.
Charoensiddhi S, Chanput WP, Sae-Tan S. Gut microbiota modulation, anti-diabetic and anti-inflammatory properties of polyphenol extract from mung bean seed coat (Vigna radiata L.). Nutrients. 2022;14(11):2275. https://doi.org/10.3390/nu14112275.
Su H, et al. Pelargonidin-3-O-glucoside derived from wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. J Agric Food Chem. 2020;68(46):13025–37.
Xing T, et al. Raspberry supplementation improves insulin signaling and promotes brown-like adipocyte development in white adipose tissue of obese mice. Mol Nutr Food Res. 2018;62(5):1701035.
Tu L, et al. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem Toxicol. 2019;133:110796.
Noratto GD, Chew BP, Atienza LM. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017;227:305–14.
Su H, et al. Pelargonidin-3-O-glucoside derived from wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. J Agric Food Chem. 2019;68(46):13025–37.
Chen X, Chen C, Fu X. Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota–mucosal barrier.” Food Funct. 2022;13(19):10121–33.
Gong P, et al. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J Ethnopharmacol. 2021;281:114558.
Wen D, et al. Astragalus mongholicus Bunge and Panax notoginseng (Burkill) FH chen formula for renal injury in diabetic nephropathy—in vivo and in vitro evidence for autophagy regulation. Front Pharmacol. 2020;11:732.
Gagliardi A, Totino V, Cacciotti F, et al. Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health. 2018;15(8):1679. https://doi.org/10.3390/ijerph15081679.
Shehadeh MB, Suaifan GARY, Abu-Odeh AM. Plants secondary metabolites as blood glucose-lowering molecules. Molecules. 2021;26(14):4333. https://doi.org/10.3390/molecules26144333.
Deka H, Choudhury A, Dey BK. An overview on plant derived phenolic compounds and their role in treatment and management of diabetes. J Pharmacopuncture. 2022;25(3):199–208.
Pérez-Burillo S, Navajas-Porras B, López-Maldonado A, Hinojosa-Nogueira D, Pastoriza S, Rufián-Henares JÁ. Green tea and its relation to human gut microbiome. Molecules. 2021;26(13):3907. https://doi.org/10.3390/molecules26133907.
Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10(11):1651.
Shabbir U, Rubab M, Daliri EB, Chelliah R, Javed A, Oh DH. Curcumin, quercetin, catechins and metabolic diseases: the role of gut microbiota. Nutrients. 2021;13(1):206. https://doi.org/10.3390/nu13010206.
Kasprzak-Drozd K, et al. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int J Mol Sci. 2021;22(7):3715.
Chen S, et al. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Mol. 2023;28(13):4982.
Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8(9):950–88.
Molinari R, Merendino N, Costantini L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: state-of-the-art. BioFactors. 2022;48(2):255–73.
Kumar Singh A, Cabral C, Kumar R, et al. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019;11(9):2216. https://doi.org/10.3390/nu11092216.
Zheng T, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/A kt/GSK 3β pathway. Br J Pharmacol. 2015;172(13):3284–301.
Pu WL, et al. Anti-inflammatory effects of Rhodiola rosea L.: a review. Biomed Pharmacother. 2020;121:109552.
Van Hul M, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol-Endocrinol Metab. 2018;314(4):E334–52.
Lee S-C, et al. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira). J Agric Food Chem. 2013;61(20):4905–13.
Shang C, Lin H, Fang X, et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021;12(24):12194–220. https://doi.org/10.1039/d1fo01935j.
Zhao H, et al. Cinnamaldehyde improves metabolic functions in streptozotocin-induced diabetic mice by regulating gut microbiota. Drug Des Dev Ther. 2021;15:2339.
Viveros A, et al. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 2011;90(3):566–78.
Fiesel A, et al. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res. 2014;10(1):1–11.
Alshaer S, et al. Changes in gut microbiota of alloxan-induced diabetic rats in response to orally administered combined aqueous extracts of olive leaves and ginger. J Appl Pharm Sci. 2022;12(3):150–9.
Samad MB, et al. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement Altern Med. 2017;17(1):1–13.
Song S, Dang M, Kumar M. Anti-inflammatory and renal protective effect of gingerol in high-fat diet/streptozotocin-induced diabetic rats via inflammatory mechanism. Inflammopharmacology. 2019;27(6):1243–54.
Zhai L, Wang X. Syringaresinol-di-O-β-D-glucoside, a phenolic compound from Polygonatum sibiricum, exhibits an antidiabetic and antioxidative effect on a streptozotocin-induced mouse model of diabetes. Mol Med Rep. 2018;18(6):5511–9.
Ojo OA, Osukoya OA, Ekakitie LI, et al. Gongronema latifolium leaf extract modulates hyperglycaemia, inhibits redox imbalance and inflammation in alloxan-induced diabetic nephropathy. J Diabetes Metab Disord. 2020;19(1):469–81. https://doi.org/10.1007/s40200-020-00533-0.
Shen X, et al. Polyphenol extracts from germinated mung beans can improve type 2 diabetes in mice by regulating intestinal microflora and inhibiting inflammation. Front Nutr. 2022;9.
Zorraquín I, et al. Current and future experimental approaches in the study of grape and wine polyphenols interacting gut microbiota. J Sci Food Agric. 2020;100(10):3789–802.
Choi BS-Y, et al. A polyphenol-rich cranberry extract protects against endogenous exposure to persistent organic pollutants during weight loss in mice. Food Chem Toxicol. 2020;146:111832.
Zhao L, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8(12):4644–56.
Yuan Y, et al. Polyphenol-rich extracts from brown macroalgae Lessonia trabeculate attenuate hyperglycemia and modulate gut microbiota in high-fat diet and streptozotocin-induced diabetic rats. J Agric Food Chem. 2019;67(45):12472–80.
Song M-Y, et al. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: a randomized, double-blind placebo-controlled study. Nutr Res. 2015;35(8):655–63.
Vetrani C, Maukonen J, Bozzetto L, et al. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020;57(7):853–60. https://doi.org/10.1007/s00592-020-01494-9.
Sharma E, Attri DC, Sati P, et al. Recent updates on anticancer mechanisms of polyphenols. Front Cell Dev Biol. 2022;10:1005910. https://doi.org/10.3389/fcell.2022.1005910.
Calabriso N, Massaro M, Scoditti E, Carluccio MA. Dietary polyphenols and their role in gut health. Nutrients. 2023;15(12):2650. https://doi.org/10.3390/nu15122650.
Lippolis T, Cofano M, Caponio GR, De Nunzio V, Notarnicola M. Bioaccessibility and bioavailability of diet polyphenols and their modulation of gut microbiota. Int J Mol Sci. 2023;24(4):3813. https://doi.org/10.3390/ijms24043813.
Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24(2):370. https://doi.org/10.3390/molecules24020370.
Gonçalves AC, Nunes AR, Falcão A, Alves G, Silva LR. dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals (Basel). 2021;14(7):690. https://doi.org/10.3390/ph14070690.
Aghababaei F, Hadidi M. Recent advances in potential health benefits of quercetin. Pharmaceuticals (Basel). 2023;16(7):1020. https://doi.org/10.3390/ph16071020.
Nishioka A, et al. Stratification of volunteers according to flavanone metabolite excretion and phase II metabolism profile after single doses of ‘Pera’ orange and ‘Moro’ blood orange juices. Nutrients. 2021;13(2).
Mukai R, et al. Chocolate as a food matrix reduces the bioavailability of galloylated catechins from green tea in healthy women. Food Funct. 2021;12(1):408–16.
Rollyson WD, et al. Bioavailability of capsaicin and its implications for drug delivery. J Control Release. 2014;196:96–105. https://doi.org/10.1016/j.jconrel.2014.09.027.
Plamada D, Vodnar DC. Polyphenols-gut microbiota interrelationship: a transition to a new generation of prebiotics. Nutrients. 2021;14(1):137. https://doi.org/10.3390/nu14010137.
Scott MB, Styring AK, McCullagh JSO. Polyphenols: bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens. 2022;11(7):770. https://doi.org/10.3390/pathogens11070770.
Martín M, Ramos S. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells. 2021;10(6).
Wen J-J, et al. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chem. 2023;404:134591.
Campos DA, Coscueta ER, Vilas-Boas AA, Silva S, Teixeira JA, Pastrana LM, Pintado MM. Impact of functional flours from pineapple by-products on human intestinal microbiota. J Funct Foods. 2020;67:103830. https://doi.org/10.1016/j.jff.2020.103830.
ClinicalTrials.gov. Type 2 diabetes intervention by gut microbiota-directed diet -a open labelled RCT, NCT05541237. 2022.
ClinicalTrials.gov, NCT05541237 type 2 diabetes intervention by gut microbiota-directed diet -a open labelled RCT (T2D). 2023
Wang S, et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes. 2022;14(1):2003176.
Mirmiranpour H, et al. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J Diabetes Metab Disord. 2020;19(1):53–60.
Sost MM, et al. A citrus fruit extract high in polyphenols beneficially modulates the gut microbiota of healthy human volunteers in a validated in vitro model of the colon. Nutrients. 2021;13(11):3915.
Sugandh F, Chandio M, Raveena F, et al. Advances in the management of diabetes mellitus: A focus on personalized medicine. Cureus. 2023;15(8):e43697. https://doi.org/10.7759/cureus.43697.
Huda MN, Kim M, Bennett BJ. Modulating the microbiota as a therapeutic intervention for type 2 diabetes. Front Endocrinol. 2021;12.
Sharifi-Rad J, Rodrigues CF, Stojanović-Radić Z, et al. Probiotics: Versatile bioactive components in promoting human health. Medicina (Kaunas). 2020;56(9):433. https://doi.org/10.3390/medicina56090433.
Blahova J, et al. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals (Basel). 2021;14(8).
Acknowledgements
The authors would like to extend their deepest gratitude to Professor Brian L. Furman, Emeritus Professor of Pharmacology, Strathclyde Institute of Pharmacy and Biomedical Sciences 161, Cathedral Street Glasgow, UK, for his diligent proofreading of this article. The authors also would like to express their gratitude to Dr. Irina Zamfir, MD, RCP London, Basildon University Hospital UK, for providing professional English editing of this manuscript and for editorial support.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sati, P., Dhyani, P., Sharma, E. et al. Gut Microbiota Targeted Approach by Natural Products in Diabetes Management: An Overview. Curr Nutr Rep (2024). https://doi.org/10.1007/s13668-024-00523-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s13668-024-00523-1