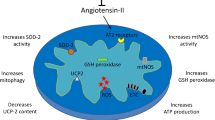
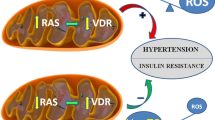
Abstract
Purpose of Review
Essential or primary hypertension (HT) is a worldwide health problem with no definitive cure. Although the exact pathogenesis of HT is not known, genetic factors, increased renin-angiotensin and sympathetic system activity, endothelial dysfunction, oxidative stress, and inflammation play a role in its development. Environmental factors such as sodium intake are also important for BP regulation, and excess sodium intake in the form of salt (NaCl, sodium chloride) increases blood pressure in salt-sensitive people. Excess salt intake increases extracellular volume, oxidative stress, inflammation, and endothelial dysfunction. Recent evidence suggests that increased salt intake also disturbs mitochondrial function both structurally and functionally which is important as mitochondrial dysfunction is associated with HT. In the current review, we have summarized the experimental and clinical data regarding the impact of salt intake on mitochondrial structure and function.
Recent Findings
Excess salt intake damage mitochondrial structure (e.g., shorter mitochondria with less cristae, increased mitochondrial fission, increased mitochondrial vacuolization). Functionally, high salt intake impairs mitochondrial oxidative phosphorylation and electron transport chain, ATP production, mitochondrial calcium homeostasis, mitochondrial membrane potential, and mitochondrial uncoupling protein function. Excess salt intake also increases mitochondrial oxidative stress and modifies Krebs cycle protein expressions.
Summary
Studies have shown that high salt intake impairs mitochondrial structure and function. These maladaptive mitochondrial changes facilitate the development of HT especially in salt-sensitive individuals.
Graphical Abstract
High salt intake impairs many functional and structural components of mitochondria. These mitochondrial alterations along with increased salt intake promote the development of hypertension.




Similar content being viewed by others
Data Availability
The papers and data supporting this review are all available as publications in public sources such as PubMed and other search engines. There is no unique empirical data generated for this review to be shared or accessed.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34.
Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–9.
Jaques DA, Wuerzner G, Ponte B. Sodium intake as a cardiovascular risk factor: a narrative review. Nutrients. 2021;13(9).
Elijovich F, Weinberger MH, Anderson CA, et al. Salt sensitivity of blood pressure: a scientific statement from the american heart association. Hypertension. 2016;68(3):e7–46.
Balafa O, Kalaitzidis RG. Salt sensitivity and hypertension. J Hum Hypertens. 2021;35(3):184–92.
• Dikalov S, Dikalova A. Mitochondrial deacetylase Sirt3 in vascular dysfunction and hypertension. Curr Opin Nephrol Hypertens. 2022;31(2):151–6. This review explains how Sirt3 is critical for vascular cell homeostasis and how the impairment of Sirt3 leads to mitochondrial dysfunction and hypertension.
Ma S, Ma L, Yang D, et al. Uncoupling protein 2 ablation exacerbates high-salt intake-induced vascular dysfunction. Am J Hypertens. 2010;23(8):822–8.
Bernal-Mizrachi C, Gates AC, Weng S, et al. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435(7041):502–6.
Tian Z, Liu Y, Usa K, et al. Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension. 2009;54(2):255–60.
Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22(2):256–65.
Iwamoto T, Kita S. Hypertension, Na+/Ca2+ exchanger, and Na+, K+-ATPase. Kidney Int. 2006;69(12):2148–54.
Babsky A, Doliba N, Doliba N, Savchenko A, Wehrli S, Osbakken M. Na+ effects on mitochondrial respiration and oxidative phosphorylation in diabetic hearts. Exp Biol Med (Maywood). 2001;226(6):543–51.
Ying WZ, Sanders PW. Cytochrome c mediates apoptosis in hypertensive nephrosclerosis in Dahl/Rapp rats. Kidney Int. 2001;59(2):662–72.
Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28(1):158–67.
Zheleznova NN, Yang C, Ryan RP, et al. Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics. 2012;44(17):829–42.
Whaley-Connell AT, Habibi J, Aroor A, et al. Salt loading exacerbates diastolic dysfunction and cardiac remodeling in young female Ren2 rats. Metabolism. 2013;62(12):1761–71.
Hernández-Ríos R, Hernández-Estrada S, Cruz-Robles D, et al. Low fructose and low salt diets increase mitochondrial DNA in white blood cells of overweight subjects. Exp Clin Endocrinol Diabetes. 2013;121(9):535–8.
He X, Liu Y, Usa K, Tian Z, Cowley AW Jr, Liang M. Ultrastructure of mitochondria and the endoplasmic reticulum in renal tubules of Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2014;306(10):F1190-1197.
Lv J, Zhang P, Zhang Y, et al. Maternal high-salt intake during pregnancy reprogrammed renin-angiotensin system-mediated cardiomyocyte apoptosis in the adult offspring heart. Reprod Sci. 2014;21(1):52–62.
Ma S, Wang Q, Zhang Y, et al. Transgenic overexpression of uncoupling protein 2 attenuates salt-induced vascular dysfunction by inhibition of oxidative stress. Am J Hypertens. 2014;27(3):345–54.
Binger KJ, Gebhardt M, Heinig M, et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest. 2015;125(11):4223–38.
Lang H, Li Q, Yu H, et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function. Br J Pharmacol. 2015;172(23):5548–58.
Wang Z, Sun Q, Sun N, Liang M, Tian Z. Mitochondrial dysfunction and altered renal metabolism in Dahl salt-sensitive rats. Kidney Blood Press Res. 2017;42(3):587–97.
Lang H, Xiang Y, Ai Z, et al. UCP3 ablation exacerbates high-salt induced cardiac hypertrophy and cardiac dysfunction. Cell Physiol Biochem. 2018;46(4):1683–92.
• Lu Z, Cui Y, Wei X, et al. Deficiency of PKD2L1 (TRPP3) exacerbates pathological cardiac hypertrophy by augmenting NCX1-mediated mitochondrial calcium overload. Cell Rep. 2018;24(6):1639–52. This study showed that a deficiency of mitochondrial TRPP3 leads to cardiac hypertrophy and hypertension.
Stocher DP, Klein CP, Saccomori AB, et al. Maternal high-salt diet alters redox state and mitochondrial function in newborn rat offspring’s brain. Br J Nutr. 2018;119(9):1003–11.
• Hasan P, Saotome M, Ikoma T, et al. Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats. J Mol Cell Cardiol. 2018;121:103–6. This study showed that in high salt conditions, dynamin-related protein1 may be involved in blood pressure elevation and blockage of dynamin-related protein1 has beneficial effects.
•• Domondon M, Polina I, Nikiforova AB, et al. Renal glomerular mitochondria function in salt-sensitive hypertension. Front Physiol. 2019;10:1588. This study showed that glomerular mitochondria in SS hypertension are functionally and structurally defective, leading to loss of podocytes and proteinuria.
Jiang L, Chen Q, Wu M, et al. Short-term high salt intake impairs hepatic mitochondrial bioenergetics and biosynthesis in SIRT3 knockout mice. Free Radic Res. 2019;53(4):387–96.
Ma T, Lin S, Wang B, et al. TRPC3 deficiency attenuates high salt-induced cardiac hypertrophy by alleviating cardiac mitochondrial dysfunction. Biochem Biophys Res Commun. 2019;519(4):674–81.
Liu Y, Yang C, Feng X, et al. Prenatal high-salt diet-induced metabolic disorders via decreasing peroxisome proliferator-activated receptor gamma coactivator 1α in adult male rat offspring. Mol Nutr Food Res. 2020;64(14): e2000196.
Woodman AG, Mah R, Keddie DL, et al. Perinatal iron deficiency and a high salt diet cause long-term kidney mitochondrial dysfunction and oxidative stress. Cardiovasc Res. 2020;116(1):183–92.
•• Geisberger S, Bartolomaeus H, Neubert P, et al. Salt transiently inhibits mitochondrial energetics in mononuclear phagocytes. Circulation. 2021;144(2):144–58. This study showed that both acute and chronic high salt intake impairs mitochondrial energy function directly.
Côrte-Real BF, Hamad I, Arroyo Hornero R, et al. Sodium perturbs mitochondrial respiration and induces dysfunctional Tregs. Cell Metab. 2023;35(2):299-315.e298.
Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–52.
Karamanlidis G, Lee CF, Garcia-Menendez L, et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18(2):239–50.
Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13(9):566–78.
Feng S, Li H, Tai Y, et al. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110(27):11011–6.
Samanta K, Mirams GR, Parekh AB. Sequential forward and reverse transport of the Na(+) Ca(2+) exchanger generates Ca(2+) oscillations within mitochondria. Nat Commun. 2018;9(1):156.
Mitchell P, Moyle J. Evidence discriminating between the chemical and the chemiosmotic mechanisms of electron transport phosphorylation. Nature. 1965;208(5016):1205–6.
Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol. 2000;529 Pt 1(Pt 1):3–10.
Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85–93.
Hernansanz-Agustín P, Choya-Foces C, Carregal-Romero S, et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020;586(7828):287–91.
Tian Z, Greene AS, Usa K, et al. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension. 2008;51(4):899–904.
He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429(6988):188–93.
Wang L, Hou E, Wang Z, et al. Analysis of metabolites in plasma reveals distinct metabolic features between Dahl salt-sensitive rats and consomic SS.13(BN) rats. Biochem Biophys Res Commun. 2014;450(1):863–869.
Usa K, Liu Y, Geurts AM, et al. Elevation of fumarase attenuates hypertension and can result from a nonsynonymous sequence variation or increased expression depending on rat strain. Physiol Genomics. 2017;49(9):496–504.
Oparil S, Acelajado MC, Bakris GL, et al. Hypertension Nat Rev Dis Primers. 2018;4:18014.
Rubattu S, Stanzione R, Volpe M. Mitochondrial dysfunction contributes to hypertensive target organ damage: lessons from an animal model of human disease. Oxid Med Cell Longev. 2016;2016:1067801.
Author information
Authors and Affiliations
Contributions
Baris Afsar drafted the work or revised it critically for important intellectual content. Rengin Elsurer Afsar contributed substantially to the conception or design of the work or the acquisition, analysis, or interpretation of data.
Corresponding author
Ethics declarations
Conflict of Interest
Both BA and REA declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afsar, B., Afsar, R.E. Mitochondrial Damage and Hypertension: Another Dark Side of Sodium Excess. Curr Nutr Rep 12, 495–507 (2023). https://doi.org/10.1007/s13668-023-00486-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00486-9