Abstract
Purpose of Review
Malignant pleural effusions (MPEs) are initially treated with thoracocentesis but usually reaccumulate. There is wide variation in the rate of recurrence. Those with rapid recurrence could benefit from early definitive treatment, whilst those with slower recurrences may not. Here, we discuss pleural fluid homeostasis, MPE pathophysiology, and factors associated with reaccumulation.
Recent Findings
Few studies have investigated markers of MPE reaccumulation. Suggested features of rapid reaccumulation include lactate dehydrogenase, effusion size, positive cytology, and dyspnoea. Vascular endothelial growth factor (VEGF) correlates with MPE size and treatment response, but its association with reaccumulation rate is unknown. Some anti-VEGF therapies have shown promise in MPE management.
Summary
Further work is needed to validate hypothesised biomarkers of rapid recurrence and to characterise other biomarkers, such as VEGF. The Reaccumulation rate of Malignant Pleural Effusions After Therapeutic Aspiration (REPEAT) study aims to address these gaps in the literature and is currently in recruitment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant pleural effusions (MPEs) are defined as fluid between the visceral and parietal pleura caused by cancer. This can be primary pleural cancer (mesothelioma) or secondary to metastatic cancer, most commonly from breast or lung. They may be the first presentation of cancer or develop in a patient with known malignancy. MPEs occur in 15% of cancer patients and are associated with a median survival of 5 months [1,2,3].
MPEs commonly cause breathlessness, chest discomfort, and cough, leading to poor quality of life. Palliation of these symptoms is key to treatment [4]. Initial treatment is often by draining fluid off, a procedure called therapeutic aspiration. This gives patients short-term symptom relief, but symptoms usually recur as the effusion reaccumulates. Patients then undergo definitive treatment. This can be insertion of an indwelling pleural catheter (IPC), a semi-permanent drain which is tunnelled under the skin, or pleurodesis, defined as artificial synthesis of the visceral and parietal pleura to obliterate the pleural space. Both treatments are effective at relieving breathlessness. Patients managed with an IPC spend less time in hospital but experience more complications. Pleurodesis is effective for about 70% of patients [5], but some need an IPC when pleurodesis fails.
MPEs form due to a disruption of normal pleural fluid production and drainage. The aim of this paper is to review the mechanisms of MPE accumulation and how rate of reaccumulation can be predicted.
Physiology of Pleural Fluid Production and Drainage in Health
Pleural fluid homeostasis is tightly regulated in physiological states, with its volume maintained at 0.1–0.3 mL/kg [6]. Pleural fluid is produced by microvascular filtration from the systemic vessels of both the parietal and visceral pleura, though the greatest contribution is from the parietal pleura. Fluid travels across the capillary endothelium, the interstitial layer, and the mesothelial membrane into the pleural space. The negative intrapleural pressure, hydrostatic, and colloid-osmotic forces across the mesothelium favour net filtration into the pleural space. It is estimated that approximately 15 ml of pleural fluid per day is produced by a healthy adult [7].
Pleural fluid is mainly reabsorbed via stomata, discrete openings of the parietal mesothelial membrane which open towards the underlying, subpleural lymphatic system. Their distribution is non-uniform, and they are only located upon the parietal pleura, with a predominance at the mediastinal and diaphragmatic pleural surfaces [8]. They facilitate the drainage of cells and particles but are not the sole effector of pleural fluid drainage. A smaller proportion of pleural fluid may be reabsorbed via other mechanisms, including electrolyte-coupled liquid outflow through the visceral and parietal mesothelium, and potentially from vesicular flow of liquid accompanying protein transcytosis [9,10,11]. There is about a 20-fold excess drainage capacity of pleural fluid drainage compared to normal physiological production [12].
Mechanisms of Pleural Fluid Production in MPE
Pleural effusions are conventionally divided into exudates and transudates. Exudates develop due to an active process and therefore have a high protein level. In contrast, transudates develop as a passive process due to disruption of colloid-osmotic pressures and have a low protein level. Though most MPEs are exudates, up to 10% are transudates, demonstrating there is not one universal mechanism of MPE production [13]. Excess pleural fluid may accumulate due to a combination of both mechanisms.
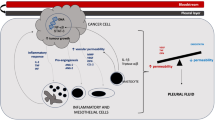
Haematogenous, metastatic spread via the pulmonary vasculature more commonly affects the visceral pleura, but can lead to subsequent tumour seeding to the parietal pleura [1, 14]. Pleural metastases instigate a local inflammatory response causing increased capillary permeability and protein leakage, with excessive fluid filtration due to colloid-osmotic forces [15, 16].
The local inflammatory reaction implicated in MPE formation has been the subject of much interest. Consequently, many cytokines, chemokines, and growth factors have been identified and are understood to play a role in MPE pathophysiology. Examples include vascular endothelial growth factor (VEGF), secreted phosphoprotein-1 (SPP-1, also called osteopontin), and transforming growth factor-B (TGF-B) [17]. In addition to the above, many other biomarkers have also been studied; however, their relationship with rate of effusion reaccumulation has rarely been considered.
Amongst the better understood biomarkers is VEGF. Although a systematic review of 20 studies demonstrated significantly higher VEGF levels in pleural fluid and serum from patients with an MPE than those with other pleural effusions, it is unclear whether greater VEGF levels are associated with faster MPE recurrence [18]. One study looking at the effects of intrapleural anti-VEGF and anti-EGFR therapies induced pleural carcinomatosis and MPEs in mice [19]. Acencio et al. found this to be universally fatal, irrespective of whether they received anti-VEGF or anti-EGFR therapies. However, the use of either therapy, individually or together, was associated with smaller MPEs seen at the 10th and 21st day of disease.
These data indicate that there is a need for further research looking at the effect of key proteins on the rate of pleural fluid production.
Impairment of Pleural Fluid Drainage in MPE
The presence of pleural metastatic disease is not sufficient for development of a pleural effusion. Given the excess drainage capacity of the normal lymphatic drainage, impairment of pleural effusion resorption via the stomata must also contribute to MPE development. This is supported by evidence from post-mortem studies demonstrating lymphatic involvement is associated with pleural effusion in metastatic disease [20]. It is thought that direct lymphatic invasion by malignant cells blocks drainage, allowing fluid to accumulate [11, 16].
Given the slow rate of pleural fluid production in health and the large excess capacity of the drainage system, it is likely that MPEs develop due to both increased pleural fluid production and impairment of drainage.
Measuring Rate of Pleural Fluid Production and Absorption
Measuring the rate of pleural fluid production in MPEs is fundamental to understanding the factors affecting it. This can be done using laboratory models or patients. Clinically, we could directly measure the rate of pleural fluid production in patients with a chest drain. In patients with an IPC, we see large variation in drainage volumes and frequency between patients, demonstrating different rates of pleural fluid production. However, the presence of an IPC itself appears to affect pleural fluid production, with a spontaneous pleurodesis occurring in about half of patients [21].
Two previous cohort studies have used time to a second pleural procedure as a surrogate for rate of production [22•, 23•]. This is a practical way of measuring this but has certain limitations. Pleural procedures may be done for diagnostic rather than therapeutic purposes. Furthermore, other factors as well as rate of pleural effusion accumulation affect the timing of pleural procedures, including clinician availability and need to stop anticoagulant medication.
In vivo animal studies have measured pleural fluid reabsorption using different methods. These studies have involved attaching small capsules to the inner surface of the rib cage of animals and inducing a hydrothorax, allowing flow from the pleural space into the capsule according to different intracapsular values [24]. Ion channel inhibitors have also been used in whole animal studies with induced hydrothoraces in order to investigate ion–coupled transportation [25].
Laboratory models have used stripped pleura from animals and humans. Pleural permeability was measured with the use of labelled particles [25] or as a response to a hydrostatic pressure difference [26]. Solute transport has also been indirectly measured in human pleura by measuring tissue resistance [27].
In the Reaccumulation rate of Malignant Pleural Effusions After Therapeutic Aspiration (REPEAT) study, we are measuring the size of the pleural effusion chest X-ray immediately following therapeutic aspiration and seven days later. Percentage opacification of the hemithorax is a validated measure of effusion size which will allow us to estimate rate of pleural fluid accumulation [28].
Variables Predicting Rate of Pleural Effusion Reaccumulation
The ability to predict the rate of pleural fluid production would enable us to plan future pleural procedures more effectively. Two previous cohort studies have looked at variables which would enable us to do this.
Boshuizen et al. undertook a prospective cohort study in 77 patients with MPE who had had therapeutic aspiration [22•]. Of these, 49 returned diaries which reported dyspnoea (at rest and exercise) for the 14 days following thoracentesis. Dyspnoea was measured at rest and exercise, using the modified Borg scale and a visual analog scale. Patients were followed up and assessed for whether re-intervention was indicated. An increase in dyspnoea from maximal relief post-procedure was most predictive of the need for further intervention (p = 0.03); however, the measure of dyspnoea at exercise was more discriminatory (p = 0.001). Of note, this was only the case when dyspnoea was measured using the modified Borg score. Similarly, patients with greater volumes of fluid drained (> 1.87 L) were more likely to need further pleural interventions (p = 0.04). Limitations of this study include that few variables were studied; a high number of patients did not complete their diaries and its relatively small size. Additionally, repeat interventions were symptom guided; hence, worsening dyspnoea would intuitively indicate a repeat procedure. This study’s findings do little to help predict whether a repeat intervention is warranted, prior to this becoming clinically apparent.
Grosu et al. performed a retrospective cohort study which studied the time to effusion recurrence necessitating intervention in 998 adults with biopsy-proven MPEs [23•]. Participants were followed up for a maximal length of 100 days following initial thoracocentesis. They found recurrence occurred by day 15 in 30% of patients and by day 90 in 48%. Size of effusion was assessed using the most recent chest X-ray (within 2 weeks prior to procedure) and was subjectively divided into four groups: blunting of costophrenic angle, up to inferior border of vascular pedicle, up to top of cardiac silhouette, and above cardiac silhouette. On univariate analysis, predictors of recurrence were solid non-lung malignancy, increased size of effusion on chest X-ray, higher pleural fluid LDH, protein level, and cholesterol. Negative cytology, clinical suspicion of pneumonia, bilateral effusions, and recent chemotherapy were associated with a lower risk. On multivariate analysis, only increasing pleural effusion size on chest X-ray (p = 0.004), volume of pleural fluid drained (p < 0.001), fluid LDH (p < 0.001), and positive cytology (p < 0.001) remained predictive of increased risk of recurrence. However, the variables identified only accounted for a small amount of the overall variability. The authors developed and externally validated a model to predict MPE recurrence in 212 patients with biopsy-proven MPEs. This performed poorly with low prediction accuracy. The authors hypothesised that this was due to differences in practice between institutions. Limitations of this study were its retrospective nature, use of a subjective and unvalidated measure of effusion size on chest X-ray, variation in time from chest X-ray to thoracentesis, and use of further intervention as a surrogate marker of rate of reaccumulation. It is interesting that despite the variation in time between chest X-ray and thoracentesis, size of effusion remained a significant predictor of further intervention.
These studies have identified that increasing breathlessness, effusion size, volume of pleural fluid drained, pleural fluid LDH, and positive cytology are predictors of need for a further drainage procedure, a surrogate marker of pleural fluid production. However, they have failed to synthesise these into a clinical tool.
Clinical Implications
The studies by Boshuizen and Grosu demonstrate that the rate of pleural effusion reaccumulation varies between patients with MPE. The ability to predict this in individual patients would allow us to inform patients more accurately and improve patient management. If a patient is aware, their symptoms will come back rapidly following therapeutic aspiration; they may choose to have an IPC inserted instead. In contrast, if the patient knows their effusion will not recur for months, they may prefer to manage it with repeated therapeutic aspiration, rather than endure a more invasive procedure. This should lead to better control of symptoms, fewer emergency admissions due to breathlessness, fewer overall invasive procedures, and a reduction in the number of cancelled procedures due to lack of pleural fluid.
A better understanding of factors driving pleural fluid production in patients with MPE may lead to new treatments which can specifically target this, reducing the need for invasive and painful procedures. At present, anti-VEGF therapies seem most promising. Higher VEGF expression is seen in patients with MPEs, and a correlation between levels and prognosis has been observed in NSCLC [29]. A study of patients receiving anti-VEGF therapies for MPEs showed those with greater reductions in serum VEGF were more likely to have longer durations of response to treatment, as determined by pleural effusion volume [30].
VEGF-based therapies include bevicuzamab and endostar. Bevacizumab is a recombinant humanised monoclonal antibody that inhibits VEGF-A [31], whilst endostar is a novel recombinant human endostatin. It utilises multiple mechanisms to exert an anti-angiogenic effect, include via the VEGF-R2 pathway [32].
Zongwen et al. conducted a meta-analysis of intrapleural bevacizumab as an adjunct to platinum-based chemotherapy for the treatment of MPEs due to lung cancer [31]. They looked at the response rate of 769 MPE patients across 11 randomised controlled trials (RCTs). A pooled estimate of the odds ratio (OR) for overall response rate was 1.40 (95% confidence interval (CI) 1.12–1.75, p = 0.003). Its use was associated with reduced chest pain, dyspnoea, and lower VEGF expression. No significant increase in adverse events was noted (OR 0.95, 95% CI 0.74–1.22). However, there is no comparison with intravenous administration.
Similarly, a meta-analysis of endostar investigated the effect of intrapleural, adjunct therapy for MPEs of any cause [32]. 13 RCTs (1066 patients) were included, and a pooled OR of the overall response rate was 3.58 (95% CI 2.73–4.69). Its use was also associated with an improved quality of life and no significant change in risk of adverse events. Again, this meta-analysis is limited by the lack of an intravenous administration comparison group.
A study comparing intravenous and intrapleural bevacizumab enrolled 43 participants and found intrapleural delivery was associated with higher efficiency and fewer adverse events. This study was significantly limited by its small sample size, and further work is warranted to compare intravenous and intrapleural treatment [30].
Additionally, these therapies may only decrease pleural fluid production, meaning they may need to be used in combination with drainage of the fluid which is already present. To the authors’ knowledge, no studies have investigated VEGF or any other biomarker’s relationship with the rate of pleural effusion reaccumulation in humans.
REPEAT Study
The REPEAT study is a currently recruiting observational cohort study of patients with known or suspected MPE undergoing therapeutic aspiration [33••]. The aim is to identify baseline variables associated with the rate of pleural effusion reaccumulation. These variables will be used to develop a clinical score, which can be used to guide management in individual patients.
There are three phases in REPEAT. The first phase is a prospective cohort study of 200 patients recruited from 10 hospitals across the UK. These patients are enrolled prior to therapeutic aspiration and baseline variables (e.g., size of pleural effusion, serum CRP, pleural fluid LDH) are recorded. Patients then undergo aspiration. They are followed up 1 week later. Rate of pleural effusion reaccumulation is estimated by comparing the size of the effusion on the post procedure chest X-ray with the size one week later. Candidate biomarkers will be assessed for their association with pleural effusion reaccumulation. These will be used to develop a model to predict rate of recurrence. Phase 2 will validate this score in a second cohort of 40 patients. Phase 3 will be a health economics and impact study, using the score in a third cohort of 200 patients, to determine its impact on key outcomes, such as breathlessness and number of pleural procedures.
Conclusion
MPEs develop due to a combination of factors including active production, passive translocation of fluid, and prevention of drainage. The relative contribution and impact of these factors will vary between patients. This leads to variation in the rate of pleural effusion reaccumulation. At present, it is not possible to predict this in individual patients. The aim of the REPEAT study is to develop a clinical score which will enable clinicians to do this accurately. This will allow us to inform our patients better, enabling them to choose timely and effective treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J. 1989 Apr;2(4):366–9.
Penz E, Watt KN, Hergott CA, Rahman NM, Psallidas I. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res. 2017;23(9):229–41.
Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69(12):1098–104.
Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010 Aug 1;65(Suppl 2):ii32–40.
Dresler CM, Olak J, Herndon JE, Richards WG, Scalzetti E, Fleishman SB, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127(3):909–15.
Yamada S, Mitarbeitern. Über die seröse Flüssigkeit in der Pleurahöhle der gesunden Menschen. 1933.
Broaddus VC. Physiology: fluid and solute exchange in normal physiological states. In: Textbook of Pleural Diseases. 2nd ed. CRC Press; 2008.
Lee YCG, Clelland CA, Rahman NM. PLEURAL SPACE. In: Laurent GJ, Shapiro SD, editors. Encyclopedia of respiratory medicine. Oxford: Academic Press. 2006;[cited 2022 Aug 18]:397–402. Available from: https://www.sciencedirect.com/science/article/pii/B0123708796005081
Miserocchi G. Mechanisms controlling the volume of pleural fluid and extravascular lung water. Eur Respir Rev. 2009;18(114):244–52.
Pistolesi M, Miniati M, Giuntini C. Pleural liquid and solute exchange. Am Rev Respir Dis. 1989;140(3):825–47.
Hatzoglou CH, Gourgoulianis KI, Molyvdas PA. Effects of SNP, ouabain, and amiloride on electrical potential profile of isolated sheep pleura. J Appl Physiol (1985). 2001 Apr;90(4):1565–9.
Jany B, Welte T. Pleural effusion in adults—etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377–86.
Ryu JS, Ryu ST, Kim YS, Cho JH, Lee HL. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18(4):230–3.
Psallidas I, Kalomenidis I, Porcel JM, Robinson BW, Stathopoulos GT. Malignant pleural effusion: from bench to bedside. Eur Respir Rev. 2016;25(140):189–98.
Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63(5):695–702.
Andrews BS, Arora NS, Shadforth MF, Goldberg SK, Davis JS. The role of immune complexes in the pathogenesis of pleural effusions. Am Rev Respir Dis. 1981;124(2):115–20.
Spella M, Giannou AD, Stathopoulos GT. Switching off malignant pleural effusion formation—fantasy or future? J Thorac Dis. 2015;7(6):1009–20.
Fafliora E, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. Systematic review and meta-analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol Rep. 2016;4(24): e12978.
Acencio MMP, Puka J, Alvarenga VA, Martins V, de Carvalho MLP, Marchi E, et al. Intrapleural targeted therapies (anti-VEGF and anti-EGFR) in the model of malignant pleural effusion. Oncotarget. 2017;8(62):105093–102.
Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21(5):437–43.
Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–9.
• Boshuizen RC, Vincent AD, van den Heuvel MM. Comparison of modified Borg scale and visual analog scale dyspnea scores in predicting re-intervention after drainage of malignant pleural effusion. Support Care Cancer. 2013 Nov;21(11):3109–16. Boshuizen et al. undertook a prospective study of 64 MPE patients to investigate features associated with the need for pleural re-intervention.
• Grosu HB, Molina S, Casal R, Song J, Li L, Diaz‐Mendoza J, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019 Jan;24(1):76–82. Grosu et al. conducted a retrospective cohort study of 988 participants with MPEs. They aimed to identify risk factors for pleural fluid recurrence following initial thoracocentesis.
Negrini D, Mukenge S, Del Fabbro M, Gonano C, Miserocchi G. Distribution of diaphragmatic lymphatic stomata. J Appl Physiol. 1991;70(4):1544–9.
Zocchi L, Agostoni E, Raffaini A. Effect on phloridzin on net rate of liquid absorption from the pleural space of rabbits. Exp Physiol. 1996;81(6):957–67.
Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiol Rev. 2004;84(2):385–410.
Kouritas VK, Tsantsaridou A, Tepetes K, Tsilimingas N, Gourgoulianis KI, Molyvdas PA, et al. Effect of histamine on the electrophysiology of the human parietal pleura. Mol Cell Endocrinol. 2011;332(1–2):271–6.
Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365(6):518–26.
Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep. 2013;15(3):207–16.
Nie K, Zhang Z, You Y, Zhuang X, Zhang C, Ji Y. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac Cancer. 2020;11(1):8–14.
Zongwen S, Song K, Cong Z, Tian F, Yan Z. Evaluation of efficacy and safety for bevacizumab in treating malignant pleural effusions caused by lung cancer through intrapleural injection. Oncotarget. 2017;8(69):113318–30.
Biaoxue R, Xiguang C, Hua L, Wenlong G, Shuanying Y. Thoracic perfusion of recombinant human endostatin (Endostar) combined with chemotherapeutic agents versus chemotherapeutic agents alone for treating malignant pleural effusions: a systematic evaluation and meta-analysis. BMC Cancer. 2016;16(1):888.
•• Reaccumulation rate of Malignant Pleural Effusions After Therapeutic Aspiration (REPEAT): an observational cohort study to create a multivariable prediction model of rate of pleural fluid reaccumulation following therapeutic aspiration in patients with malignant pleural effusion attending a pleural clinic - NIHR Funding and Awards. [cited 2022 Aug 7]. Available from: https://fundingawards.nihr.ac.uk/award/NIHR201466. The REPEAT study is currently in enrolment. Mishra et al. aim to identify biomarkers related to MPE recurrence and to develop a clinical score. This will be validated as a predictive tool for MPE recurrence following thoracocentesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors of this paper do not have any conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pleural Diseases and Mesothelioma.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sethi, D.K., Kouritas, V. & Mishra, E. Factors Affecting Rate of Pleural Fluid Accumulation in Patients with Malignant Pleural Effusions. Curr Pulmonol Rep 12, 10–15 (2023). https://doi.org/10.1007/s13665-023-00299-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-023-00299-9