Abstract
Accurate mediastinal lymph node staging is critical for patients with non-small cell lung cancer (NSCLC). Although cervical mediastinoscopy has been regarded as the method of choice for mediastinal staging in NSCLC, emergence of minimally invasive sonography, for example endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound fine needle aspiration (EUS-FNA), has led to questions about the routine use of mediastinoscopy. EBUS-TBNA has access to all the mediastinal lymph nodes accessible by mediastinoscopy but also extends to the N1 nodes. EUS-FNA enables access to paraesophageal (station 8) and pulmonary ligament (station 9) lymph nodes, which are not accessible by either mediastinoscopy or EBUS-TBNA. On the basis of current evidence, sonographic staging is the recommended choice for patients with high pretest probability of lymph node metastatic involvement; all negative results should, however, be verified by mediastinoscopy, especially in centers with low expertise. For patients with low pretest probability, mediastinoscopy may be omitted.
Similar content being viewed by others
Introduction
Mediastinal staging determines therapy in non-small cell lung cancer (NSCLC). Patients with metastases to the mediastinal lymph nodes (N2 or N3) are usually treated with chemotherapy and/or radiation whereas those without involvement of the mediastinum (N0 or N1) are usually treated by surgical resection. Mediastinoscopy is part of routine staging at many institutions and has traditionally been regarded as the method of choice for invasive staging of the mediastinum [1]. The development of less invasive techniques, for example endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound fine needle aspiration (EUS-FNA), coupled with improvement of non-invasive imaging by computed tomography (CT) and positron emission tomography (PET), has led to questions about the routine use of mediastinoscopy [2–5]. This article reviews the indications for invasive staging of the mediastinum in NSCLC, with special emphasis on the ideal modality of staging in different clinical scenarios.
Non-invasive mediastinal staging
Non-invasive radiological staging of the mediastinum should be undertaken for every patient with diagnosis or suspicion of NSCLC [6]. A CT scan of the chest with intravenous contrast provides important information about the tumor and associated hilar or mediastinal lymph nodes [7]. CT criteria for suspicious lymph nodes include a short-axis diameter of >1 cm on transverse images or evidence of central necrosis [5, 7]. PET scan should also be performed to characterize the primary tumor and eliminate the possibility of distant metastatic disease. If the primary tumor is avid to fluorodeoxyglucose (FDG), a PET scan provides insight on the status of mediastinal and hilar lymph nodes [8]. Bearing in mind that the standardized uptake value (SUV) is non-uniform among different institutions, a cutoff SUV of greater than 2.5 has been used to indicate suspicious lymphadenopathy on PET scan [9].
Because of the incidence of false-positive imaging, it is generally accepted that any suggestion of suspicious lymphadenopathy on CT or PET should result in prompt invasive mediastinal staging to seek pathological confirmation of lymph node spread [10]. In contrast, because the incidence of false negative results is low (3–9 %) for combined CT/PET, invasive mediastinal staging can be omitted in cases where both CT and PET fail to provide any evidence of suspicious lymphadenopathy [4, 5]. There is general agreement that a patient with a peripheral T1a tumor, negative clinical evaluation, and negative CT/PET can proceed to surgery without preoperative invasive mediastinal staging, if lymph node sampling is performed during the surgical procedure [10].
Invasive mediastinal staging
Invasive staging of the mediastinum is indicated in most cases of lung cancer when resection with curative intent is contemplated, unless the patient presents with a peripheral clinical stage T1aN0M0 lesion. Invasive staging is especially indicated when preoperative imaging is suggestive of N1, N2, or N3 disease, when dealing with central tumors, or when the tumor has low SUV on PET scan [11]. In the modern era, invasive staging is performed by mediastinoscopy, EBUS-TBNA, EUS-FNA, or a combination thereof.
Mediastinoscopy
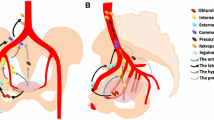
Cervical mediastinoscopy is performed under general anesthesia in the operating room, but usually does not require post-operative hospitalization. It involves a small neck incision above the sternal notch and access to the pretracheal fascia. After blunt digital dissection and palpation of the mediastinal vessels, the mediastinoscope is inserted, and blunt dissection is conducted at the nodal stations of interest. Samples from the upper and lower paratracheal (stations 2R, 2L, 4R, 4L) and anterior subcarinal (station 7) nodes should be obtained at every mediastinoscopy. Posterior subcarinal (station 7), aorto-pulmonary window (stations 5, 6), and inferior mediastinal (stations 8, 9) nodes are not accessible by the mediastinoscope and cannot be sampled [12]. After hemostasis, the wound is closed in layers.
Mediastinoscopy has long been regarded as the method of choice for invasive mediastinal staging, and, by some guidelines, is recommended before surgical resection of lung cancer with curative intent [5]. Although it is usually a safe procedure with a low incidence of complications (1.5–3 %) [13, 14], mediastinoscopy still carries a risk of devastating consequences from vascular or recurrent laryngeal nerve injury, requires the resources of the operating room, and is accompanied by all the implications of general anesthesia. Contraindications to mediastinoscopy include severe cervical arthritis or cervical instability limiting neck extension and cutaneous tracheostomy.
The range of reported sensitivity of mediastinoscopy varies from 40–92 % [5], probably because mediastinoscopy performed by an inexperienced surgeon has a much lower lymph node yield than that performed by a thoracic surgeon who treats lung cancer [15, 16]. Recent systematic reviews have narrowed the range of sensitivity to 78–81 %, but it is important to note that in many of the studies analyzed the prevalence of lung cancer was as low as 39 % [3]. More importantly, mediastinoscopy is an excellent test to discount the possibility of mediastinal involvement in lung cancer, with reported specificity of 100 % and negative predictive value of 91 % [5]. Mediastinoscopy will be negative for a patient who has involvement of the aorto-pulmonary lymph nodes (Stations 5, 6), the prevascular nodes (Station 3), the inferior pulmonary ligament nodes (Station 9), and the periesophageal nodes (Station 8), all of which are not accessible by the mediastinoscope [17].
EBUS-TBNA
Convex probe endobronchial ultrasound (CP-EBUS) with capability of real-time EBUS-TBNA was first introduced in 1994 [18] and quickly became prominent in lymph node staging of lung cancer [18]. The dedicated ultrasound scanner produces images of excellent quality, with power and color Doppler available for precise characterization of mediastinal blood vessels. Twenty-one or 22-gauge needles can be used for real-time endobronchial ultrasound-guided transbronchial tissue aspiration from mediastinal lymph nodes. Similar to mediastinoscopy, CP-EBUS can access the upper paratracheal (Stations 2R, 2L), lower paratracheal (Stations 4R, 4L), and subcarinal (Station 7), lymph nodes, but can also extend further to gain access to the intrapleural hilar (Station 10), interlobar (Station 11), and lobar (Station 12) nodal stations. CP-EBUS cannot gain access to the prevascular (Station 3a), sub-aortic (Station 5), para-aortic (Station 6), paraesophageal (Station 8), or pulmonary ligament (Station 9) nodes. Some authors have used the CP-EBUS endoscope through the esophagus to sample the paraesophageal (Station 8) and the pulmonary ligament (Station 9) nodes [19]
EBUS-TBNA has reported sensitivity of 94–98 %, specificity of 100 %, positive predictive value of 100 %, negative predictive value of 89.5 %, and diagnostic accuracy of 96.3 % in the mediastinal staging of lung cancer [20]. Studies have also shown that results of EBUS-TBNA can significantly affect treatment decisions in the management of lung cancer. In one prospective study of 105 patients, EBUS-TBNA avoided 29 mediastinoscopies, eight thoracotomies, four thoracoscopies, and nine CT-guided percutaneous biopsies [21]. The largest multicenter prospective study to date evaluated 502 lung cancer patients with documented mediastinal and hilar lymphadenopathy [20]. The safety, efficacy, and high diagnostic yield of EBUS-TBNA have been proven in several large series and meta-analyses, especially in patients with radiological evidence of enlarged mediastinal lymph nodes [9, 21–25]. On the basis of this evidence, EBUS-TBNA was included in the second edition of the American College of Chest Physicians’ evidence-based practice guidelines for the invasive staging of lung cancer [26].
Complications related to EBUS-TBNA are similar to those of conventional TBNA including pneumothorax, pneumomediastinum, hemomediastinum, mediastinitis, bacteremia, and pericarditis. No major complications of EBUS-TBNA have been reported in the literature [27].
EUS-FNA
EUS-FNA is also becoming an important adjunct in the staging of lung cancer, especially because it can sample lymph node stations which are not accessible by mediastinoscopy or EBUS [28]. The dedicated ultrasound scanner, mounted on a side viewing videogastroscope enables tissue visualization at a radius of 2–10 cm around the esophagus. Transesophageal real-time ultrasound-guided biopsies of mediastinal structures are obtained by use of an 80 mm long needle with a diameter of 22, 21, or 19-gauge. As the gastroscope is retracted from the stomach into the cervical esophagus, the inferior pulmonary ligament (Station 9), paraesophageal (Station 8), subcarinal (Station 7, especially posterior), and paratracheal (Stations 2, 4) nodes are visualized and biopsied.
The sensitivity of EUS-FNA has been reported to range between 74 and 92 % [29–31]. Two recent meta-analyses, combining more than 1000 patients have determined the sensitivity to be closer to 83–84 % [5, 25]. Unlike for other modalities, the sensitivity of EUS-FNA seems to be affected by variables such as node size, location of the tumor, and nodal station. One study showed that sensitivity can be as high as 91.7 % for bulky lymph nodes and as low as 43.8 % for nodes smaller than 1 cm on CT scan [32]. It was also shown that sensitivity for right-sided tumors (50 %) was lower than for left-sided tumors (96.6 %), and that sensitivity was highest (80.6 %) for station 7 and lowest (23.8 %) for station 4R.
Unlike mediastinoscopy and EBUS-TBNA, EUS-FNA is reported to have a low NPV of 73 %, and is not a very reliable test for precluding the possibility of lymph node spread. Most authors agree that negative results of EUS-FNA should be verified by another means, especially in the presence of suggestive imaging [28].
Combined ultrasonography (CUS)
CUS combines EBUS-TBNA and EUS-FNA for staging of the mediastinum, ensuring that the lymph nodes that are not accessible by one technique are accessed by the other. With CUS, all the mediastinal lymph node stations are biopsied, with some authors even reporting successful sampling of aorto-pulmonary nodes (Stations 5/6) [28]. In the original study on CUS, Vilmann et al. demonstrated excellent diagnostic yield and 100 % accuracy in detecting nodal metastases. Subsequent to that, other studies have reproduced results with sensitivity ranging between 91.1 and 93 % accuracy of 91–97 %, and NPV of 91–95 % [19, 33–35]. As such, CUS has been shown to be more sensitive than EBUS or EUS separately, with a significantly better NPV [36].
Mediastinoscopy versus ultrasonography
The emergence of EBUS-TBNA and EUS-FNA was accompanied by the emergence of a debate about whether ultrasonography should replace or complement mediastinoscopy in invasive mediastinal staging for lung cancer. Although many studies have shown that ultrasonography achieves better sensitivity, accuracy, and NPV than historical mediastinoscopy, few studies have compared the two modalities in a prospective controlled fashion.
In a prospective study of 153 patients, EBUS-TBNA followed by mediastinoscopy were performed on all patients in a blinded fashion, in which the surgeon performing one technique was unaware of the results of the other [37•]. EBUS-TBNA was able to sample an equivalent amount of lymph nodes compared with mediastinoscopy and there was excellent agreement between the two techniques for 91 % of patients (Kappa = 0.8). The specificity and positive predictive value of both tests were 100 %. The sensitivity, negative predictive value, and diagnostic accuracy of mediastinal lymph node staging for EBUS-TBNA and mediastinoscopy were 81 %, 91 %, 93 %, and 79 %, 90 %, 93 %, respectively, without any statistically significant differences. The authors were able to demonstrate that in a setting in which experienced thoracic surgeons perform the procedure, and where rapid on-site pathological examination is available, EBUS-TBNA is equivalent to mediastinoscopy for staging of the mediastinum.
In another prospective study of 60 patients, the authors sought to compare the results of mediastinoscopy to those of EUS-FNA [38]. In the EUS-FNA group, the results of endosonographic staging were verified by thoracotomy or mediastinoscopy. The sensitivity of EUS-FNA in the right paratracheal (4R) region was 67 %, versus 33 % for mediastinoscopy. In the left paratracheal (4L) region, EUS-FNA was also more sensitive than mediastinoscopy (80 % vs. 33 %). Most strikingly, the sensitivity of EUS-FNA at the subcarinal station (7) was 100 %, versus only 7 % for mediastinoscopy, probably because of the ability of EUS-FNA to visualize and sample the postero-inferior aspect of the subcarinal nodes, an area not accessible to the mediastinoscope. The authors concluded that EUS-FNA is better than mediastinoscopy because not only does it sample lymph node stations not accessible by mediastinoscopy but it is also more sensitive at the lymph node stations that are accessible by both tests.
In a multi-center randomized trial comparing 118 patients who had mediastinoscopy alone with 123 patients who had combined ultrasonography followed by mediastinoscopy, if the results of ultrasonography were negative, the authors were able to show that combined staging with mediastinoscopy and endosonography is better than mediastinoscopy alone [39••]. Of the 123 patients who started with endosonography, 67 patients actually underwent mediastinoscopy, with nodal metastases being missed for four patients and corresponding sensitivity of 94 % and NPV of 93 %. In the mediastinoscopy only group, sensitivity was 80 % (p = 0.04) and the NPV was 86 %, which was statistically no different from use of the combined approach (p = 0.26). Despite the lack of difference in NPV, the study demonstrated that the number of unnecessary thoracotomies was reduced by half in the combined approach group, which led the authors to recommend a new staging strategy of choice which begins with combined endosonography and then proceeds to mediastinoscopy if lymph node metastasis is not demonstrated.
When should mediastinoscopy be performed?
The debate on the modern indications of mediastinoscopy continues to evolve, and it is likely that the future of mediastinal staging lies in a combination of mediastinoscopy with minimally invasive techniques. At present, not all institutions that practice thoracic surgery have the equipment, expertise, or trained personnel to safely and reliably perform sonographic staging. Mediastinoscopy is a time-proven technique that is part of the armamentarium of any thoracic surgeon, and should be the invasive method of choice for staging the mediastinum in settings in which sonographic staging is not available or is suboptimum.
The debate becomes more significance at institutions with equipment for EBUS-TBNA or EUS-FNA, the operator expertise, and the trained personnel to reliably perform these procedures as demonstrated in the aforementioned studies. It is clear that not all lymph nodes can be sampled by one procedure alone, and, therefore, consideration of the clinical scenario is important before deciding on the best approach to invasive mediastinal staging. It is also evident from the data that the sensitivity of each of those techniques is enhanced by the larger size of the lymph nodes and the pre-test probability of lymph node metastases [40]. Hence, determining this pre-test probability based on CT scan of the chest can categorize lung cancer patients into four broad groups which could be approached differently [5, 41] (Fig. 1).
Group I: bulky mediastinal lymphadenopathy or infiltration
These patients are usually diagnosed with mediastinal spread on the basis of radiographic evidence, and extensive invasive staging is not required. A biopsy of involved lymph nodes should be obtained to confirm diagnosis or guide therapy, and this is usually done from whatever location is easiest using the corresponding highest-yield technique. In such a scenario, sonographic techniques will usually take precedence over mediastinoscopy, because of the high likelihood of obtaining a diagnosis.
Group II: discrete enlarged mediastinal lymph nodes
Patients who have radiographically positive lymph nodes should undergo confirmation of disease by invasive testing, because false-positive results for chest CT and PET range between 15 and 40 % [40]. In this case, the first variable affecting the choice of technique is the location of the suspicious lymph node. For example, station 8 or 9 nodes are only approachable by EUS and stations 10 or beyond are only approachable by EBUS-TBNA. Station 7 yields are better with EBUS-TBNA and EUS-FNA, and, therefore, should be approached sonographically. The second variable is the availability of the equipment, technical expertise, and level of interest that are necessary to achieve the performance characteristics of sonographic staging, as reported in the literature. If the location is favorable and the technology is available, these patients should be staged sonographically with EUS-FNA, EBUS-TBNA, or CUS. If lymph node spread is demonstrated, these patients should be referred to chemo-radiation treatment. If, on the other hand, lymph node spread is not demonstrated by sonographic techniques, these patients should undergo mediastinoscopy to confirm the absence of disease, because, for such patients, the incidence of false negative results for FNA can approach 30 % [40].
Group III: normal mediastinal lymph nodes, but evidence of central tumors or N1 lymph node involvement
Patients with normal mediastinal lymph nodes, but with evidence of central tumors or hilar lymph node involvement carry a 20 % chance of harboring radiographically occult N2 disease. For this patient population, the incidence of false-negative results for mediastinoscopy is low, ranging from 7–9 %, and this technique is still accepted as the standard for mediastinal staging [42, 43]. Because the mediastinal lymph nodes are normal, EUS-FNA is not recommended as the only modality of staging, because of the reported high incidence of false-negative results, ranging between 14 and 21 % [5, 44–46]. In expert hands, EBUS-TBNA is able to achieve acceptable results, especially if the nodes range between 0.5 and 1 cm in size [27]. EBUS-TBNA also has the unique advantage of being able to sample the N1 nodes (Stations 10, 11, and 12), which can provide valuable diagnostic and therapeutic information to guide treatment. Therefore, these patients can be approached initially with EBUS-TBNA or CUS. If lymph node metastasis is not demonstrated then mediastinoscopy is generally recommended to confirm diagnosis, unless the level of expertise with EBUS-TBNA is equivalent to that in published studies. Also acceptable for staging would be to proceed straight to mediastinoscopy.
Group IV: normal mediastinal lymph nodes, normal N1 lymph nodes, and peripheral tumors
For patients with small peripheral tumors, the incidence of false-negative results for chest CT is 10 % [45] and that for CT/PET can be as low as 5 % [47]. It can be argued that invasive staging by mediastinoscopy is not recommended for this group, because the incidence of false-negative results for mediastinoscopy is higher than for non-invasive staging. Existing guidelines recommend omitting preoperative invasive staging for patients with clinical stage T1aN0M0 if mediastinal lymph node sampling is conducted during the surgical procedure [10]. For patients with tumors larger than T1a, invasive staging can be performed by EBUS-TBNA, EUS-FNA, or CUS. If lymph node metastasis is not demonstrated, these patients can proceed directly to surgery, without undergoing mediastinoscopy.
Conclusion
The emergence of EBUS-TBNA and EUS-FNA was accompanied by the emergence of a debate about whether ultrasonography should replace, or complement, mediastinoscopy in invasive mediastinal staging for lung cancer. Studies have demonstrated that the results of ultrasonographic staging are highly dependent on the pretest probability of lymph node metastasis and the level of operator and institutional expertise. Mediastinoscopy should be the invasive method of choice for staging the mediastinum in settings in which sonographic staging is not available or not optimum. For patients with high pretest probability of lymph node spread, sonographic staging is recommended as a starting point, but all negative results should be verified by mediastinoscopy, especially in centers with low expertise. For patients with low pretest probability, mediastinoscopy can be omitted.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg. 1999;118(5):894–9.
Yasufuku K, Fujisawa T. Staging and diagnosis of non-small cell lung cancer: Invasive modalities. Respirology. 2007;12(2):173–83.
Toloza EM, Harpole L, McCrory DC. Noninvasive Staging of Non-Small Cell Lung Cancer. Chest. 2003;123(1):137S–46S.
De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32(1):1–8.
Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):202S–20S.
Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. The Noninvasive Staging of Non-Small Cell Lung Cancer. Chest. 2003;123(1):147S–56S.
Groth SS, Whitson BA, Maddaus MA. Radiographic Staging of Mediastinal Lymph Nodes in Non–Small Cell Lung Cancer Patients. Thoracic Surgery Clinics NA. 2008;18(4):349–61.
Freudenberg LS, Rosenbaum SJ, Beyer T, Bockisch A, Antoch G. PET Versus PET/CT Dual-Modality Imaging in Evaluation of Lung Cancer. Thoracic Surgery Clinics NA. 2010;20(1):25–30.
Herth FJF, Annema JT, Eberhardt R, et al. Endobronchial Ultrasound With Transbronchial Needle Aspiration for Restaging the Mediastinum in Lung Cancer. J Clin Oncol. 2008;26(20):3346–50.
Darling GE, Dickie AJ, Malthaner RA, Kennedy EB, Tey R. Invasive mediastinal staging of non-small-cell lung cancer: a clinical practice guideline. Curr Oncol. 2011;18(6):e304–10.
Rami-Porta R, Call S. Invasive Staging of Mediastinal Lymph Nodes: Mediastinoscopy and Remediastinoscopy. Thoracic Surgery Clinics NA. 2012;22(2):177–89.
Lerut T, De Leyn P, Coosemans W, et al. Cervical Videomediastinoscopy. Thoracic Surgery Clinics NA. 2010;20(2):195–206.
Luke WP, Pearson FG, Todd TR, Patterson GA, Cooper JD. Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg. 1986;91(1):53–6.
Kirby T, Fell S. Mediastinoscopy. In: Pearson FG, Cooper JD, Deslauriers J, editors. Thoracic Surgery. New York: Churchill Livingstone; 2002. p. 98–103.
Little AG, Rusch VW, Bonner JA, et al. Patterns of Surgical Care of Lung Cancer Patients. Ann Thorac Surg. 2005;80(6):2051–6.
Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135(2):247–54.
Yasufuku K, Keshavjee S. Staging Non–Small Cell Lung Cancer. Clin Pulm Med. 2010;17(5):223–31.
Yasufuku K, Chhajed PN, Sekine Y, et al. Endobronchial ultrasound using a new convex probe: a preliminary study on surgically resected specimens. Oncol Rep. 2004;11(2):293–6.
Herth FJF, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138(4):790–4.
Herth FJF, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61(9):795–8.
Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50(3):347–54.
Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130(3):710–8.
Hwangbo B, Kim SK, Lee H-S, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest. 2009;135(5):1280–7.
Gu P, Zhao Y-Z, Jiang L-Y, Zhang W, Xin Y, Han B-H. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur J Cancer. 2009;45(8):1389–96.
Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax. 2009;64(9):757–62.
Health ACOCP, Committee SP. Diagnosis and management of lung cancer: ACCP evidence-based guidelines. American College of Chest Physicians. Chest. 2003;123(1 Suppl):1–337.
Yasufuku K. Current clinical applications of endobronchial ultrasound. Expert Rev Respir Med. 2010;4(4):491–8.
Kużdżał J, Szlubowski A. Ultrasound-Guided Transbronchial and Transesophageal Needle Biopsy in the Mediastinal Staging of Lung Cancer. Thoracic Surgery Clinics NA. 2012;22(2):191–203.
Nguyen TQ, Kalade A, Prasad S, et al. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) of mediastinal lesions. ANZ J Surg. 2011;81(1–2):75–8.
Talebian M, von Bartheld MB, Braun J, et al. EUS-FNA in the preoperative staging of non-small cell lung cancer. Lung Cancer. 2010;69(1):60–5.
Annema JT, Versteegh MI, Veseliç M, Voigt P, Rabe KF. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol. 2005;23(33):8357–61.
Witte B, Neumeister W, Huertgen M. Does endoesophageal ultrasound-guided fine-needle aspiration replace mediastinoscopy in mediastinal staging of thoracic malignancies? Eur J Cardiothorac Surg. 2008;33(6):1124–8.
Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal Endoscopic Ultrasound-Guided Fine- Needle Aspiration (EUS-FNA) and Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) Biopsy: a Combined Approach in the Evaluation of Mediastinal Lesions. Endoscopy. 2005;37:833–9.
Hwangbo B, Lee G-K, Lee H-S, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010;138(4):795–802.
Wallace MB, Pascual JMS, Raimondo M, et al. Minimally Invasive Endoscopic Staging of Suspected Lung Cancer. JAMA. 2008;299(5):540–6.
Szlubowski A, Zielinski M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging- a prospective trial. Eur J Cardiothorac Surg. 2010;37(5):1175–9.
• Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142(6):1393–400. e1. The first prospective study to compare the diagnostic yield of mediastinoscopy and EBUS-TBNA, for mediastinal staging, for patients with potentially resectable NSCLC.
Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy versus mediastinoscopy for analysis of paratracheal and subcarinal lymph nodes in lung cancer staging. Lung Cancer. 2005;48(1):85–92.
•• Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304(20):2245–52. The first multi-center randomized controlled trial to compare combined endoscopic (EUS-FNA+EBUS-TBNA) and surgical staging with surgical staging alone.
Schipper P, Schoolfield M. Minimally Invasive Staging of N2 Disease: Endobronchial Ultrasound/Transesophageal Endoscopic Ultrasound, Mediastinoscopy, and Thoracoscopy. Thoracic Surgery Clinics of NA. 2008;18(4):363–79.
Coughlin M, Deslauriers J, Beaulieu M, et al. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. ATS. 1985;40(6):556–60.
Choi YS, Shim YM, Kim J, Kim K. Mediastinoscopy in patients with clinical stage I non-small cell lung cancer. ATS. 2003;75(2):364–6.
Gürses A, Turna A, Bedirhan MA, et al. The value of mediastinoscopy in preoperative evaluation of mediastinal involvement in non-small-cell lung cancer patients with clinical NO disease. Thorac Cardiovasc Surg. 2002;50(3):174–7.
Wallace MB, Silvestri GA, Sahai AV, et al. Endoscopic ultrasound-guided fine needle aspiration for staging patients with carcinoma of the lung. ATS. 2001;72(6):1861–7.
Detterbeck FC. Integration of Mediastinal Staging Techniques for Lung Cancer. Semin Thorac Cardiovasc Surg. 2007;19(3):217–24.
LeBlanc JK, Devereaux BM, Imperiale TF, et al. Endoscopic ultrasound in non-small cell lung cancer and negative mediastinum on computed tomography. Am J Respir Crit Care Med. 2005;171(2):177–82.
Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography– and positron emission tomography–screened patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2006;131(4):822–9. e2.
Disclosure
W.C. Hanna: none; K. Yasufuku: educational and research support funding from Olympus Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanna, W.C., Yasufuku, K. Mediastinoscopy in the era of endobronchial ultrasound: when should it be performed?. Curr Respir Care Rep 2, 40–46 (2013). https://doi.org/10.1007/s13665-012-0032-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-012-0032-y