Abstract
Bioactive compounds from the wood-decay fungus Xylaria cf. longipes SWUF08-81, cultivated in three different culture media (GM, YM and PDB), were isolated. Their structures and stereochemistry were deduced from spectroscopic and MS data analysis, together with quantum chemical calculations of 13C NMR chemical shifts and electronic circular dichroism (ECD) spectra. Five undescribed polyketides including dibenzofuran (1), mellein (2), dihydroisocoumarin (15), and two pyrans (16, 17), together with twenty-three compounds were determined. Compounds 18 and 20 were significantly toxic against cancer cell lines (HCT116, HT29, MCF-7 and HeLa) based on the MTT assay. Quantification by HPLC showed that 18 was produced three-fold higher in the broth of PDB than YM. These studies showed that the production of different compounds were primarily dependent on nutrition sources and it has given a starting point for the growth optimization conditions for the scaling up of bioactive compounds production.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fungi are an exceptional bioactive compounds producer, many of which have been developed as drugs or drug candidates [1, 2]. The diversity of fungi metabolites is greatly influenced by the growth environment, which includes geographical location and nutrition. Examining growth conditions such as carbon and nitrogen sources, pH, temperature, stress, extreme environment and co-culture in culture media allowed for the establishment of quantity and quality profiles for compounds of interest [3,4,5]. The manufacture of high quantity bioactive compounds in a short period was made possible by controlling the cultivation conditions. It is for these reasons that fungi continues to be a vital source for potential drugs discovery and development [6].
The Xylariaceae family belongs to Ascomycota, known as wood-decay fungi and found throughout tropical regions [7]. Xylaria is one of the genera in Xylariaceae that has been studied and well documented to produce a wide range of cytotoxic compounds [8,9,10]. A number of previous research studies revealed that various bioactive compounds from this genus had evolved from habitat, associated climate and the complexity of the fungi species [11,12,13,14].
To continue the search for potential anticancer drugs candidates from the Xylaria genus, the chemical constituents from Xylaria cf. longipes SWUF08-81 in three different culture media (GM, YM and PDB) and the quantities of selected active compounds were validated. A few reports on Xylaria longipes showed various bioactive compounds that gave some insights into the continuing search for bioactive compounds in different environments [13,14,15].
Herein, we have described the results of the purification, structure elucidation, and evaluation of antiproliferative activity against cancer cells (HCT116, HT29, MCF-7 and HeLa), and normal Vero cells. Together with the quantification of cytotoxic compounds through the high-performance liquid chromatography (HPLC) method using a diode array detector (DAD).
2 Results and discussion
2.1 Purification and structure elucidation
The X. cf. longipes SWUF08-81 fungus was cultivated in three different media named GM, YM and PDB media. The compounds were extracted from broth and mycelium by organic solvents and the crude extracts were purified by chromatographic separation. Fourteen compounds (1–14) were isolated from GM medium, and fourteen compounds (15–28) were isolated from YM medium. Eight compounds were isolated from PDB medium, all of which were also identified from the YM medium, including compounds 17, 18–22, 24 and 26.
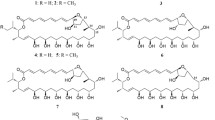
The analysis of spectroscopic and MS data, in combination with the 13C NMR chemical shift and the quantum chemical ECD calculations [16, 17], revealed two undescribed polyketides 1,3,8-trihydroxy-7-methoxy-9-methyldibenzofuran (1) and (3R)-7-methoxy-5-methoxycarbonylmellein (2) form GM medium, together with twelve known compounds including (−)-mellein (3) [18], (−)-5-methoxycarbonyl- mellein (4) [19], (−)-5-carboxymellein (5) [19], cytochalasin D (6) [19], zygosporin D (7) [20], 19,20-epoxycytochalasin D (8) [21], cytochalasin Ohyp (9) [22], cytochalasin C (10) [22], 2-chloro-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione (11) [23], 2-hydroxy-5-methoxy-3-methyl cyclo-hexa-2,5-diene-1,4-dione (12) [24], 4-hydroxy-methylbenzoate (13) [25] and (4R,5S,6R)-4,5,6-trihydroxy-3-methoxy-5-methyl-cyclohex-2-n-1-one (14) [23]. There were three undescribed compounds, including dihydroisocoumarin, (3R,2′R,6′R)-asperentin-8-O-methylether (15) and two pyran derivatives, (6S,2′R,6′S)-6-methyl-2-((6-methyltetrahydro-2H-pyran-2-yl)methyl)-2,3-dihydro-4H-pyran-4-one (16) and (2′R,6′S)-5-((-6-methyltetrahydro-2H-pyran-2-yl)methyl)benzene-1,3-diol (17) from YM, together with eleven known compounds including five dihydroisocoumarins, asperentin (18) [26, 27], asperentin-8-O-methylether (19) [27, 28], asperentin-6-O-methylether (20) [26], 5′-hydroxyasperentin (21) [26] and 4′-hydroxyasperentin (22) [26], four auroglaucins, tetrahydroauroglaucin (23) [29], flavoglaucin (24) [29], auroglaucin (25) [29] and isodihydroauroglaucin (26) [29], alkaloid, echinulin (27) [30], and anthraquinone, physcion (28) derivatives [31] (Fig. 1).
Compound 1 was a brown amorphous solid with a melting point of 224–225 °C. Its molecular formula of C14H12O5 was deduced from the [M + Na]+ peak at m/z 283.0597 in the HRESIMS (calcd. for C14H12O5Na+, 283.0577). The key IR spectrum showed absorption bands of O–H stretching at 3354 cm−1 and aromatic C = C stretching at 1615, 1509 and 1462 cm−1. The 1H NMR data (Table 1) displayed proton signals of an aromatic at δH 6.92 (1H, s, H-6), 6.40 (1H, d, J = 1.7 Hz, H-4) and 6.22 (1H, d, J = 1.7 Hz, H-2), a methoxy at δH 3.91 (3H, s, H-7-OCH3), and a methyl at δH 2.77 (3H, s, H-9-CH3). The 13C NMR data (Table 1) showed signals of aromatic carbons at δC 159.1 (C-3), 156.6 (C-4a), 152.0 (C-1), 149.1 (C-5a), 145.8 (C-7), 140.1 (C-8), 117.6 (C-9), 116.5 (C-9a), 106.1 (C-9b), 97.0 (C-2), 91.7 (C-6) and 89.4 (C-4). There was a methoxy carbon signal at δC 55.3 (C-7-OCH3) and a methyl carbon signal at 13.2 (C-9-CH3). There was no COSY correlation observed in 1, therefore the structure was deduced from key HMBC correlations between H-2 to C-1, C-4 and C-9b, and H-4 to C-2, C-3, C-4a and C-9b, confirming the tetrasubstituted aromatic ring A. The aromatic ring C was indicated by the correlations between H-6 to C-5a, C-7, C-8 and C-9a, and 9-CH3 to C-8, C-9 and C-9a. When compared to those in literature, the NMR data of 1 suggested that 1 shared a dibenzofuran scaffold similar to the known 2,7-dihydroxy-1,6-dimethoxy-9-methyldibenzofuran [32, 33]. The complete structure was confirmed by the NOE experiment of the methylated product, 1a (Fig. 2). The correlation between H-1-OCH3 and H-2, H-3-OCH3 and H-4, H-7-OCH3 and H-6, and H-8-OCH3 and H-9-CH3 confirmed the methoxy positions on both aromatic rings. Therefore, 1 was deduced as a new dibenzofuran, named 1,3,8-trihydroxy-7-methoxy-9-methyldibenzofuran.
Compound 2 was a white amorphous solid with a melting point of 135–136 °C. The [M + Na]+ peak at m/z 289.0687 in the HRESIMS indicated a molecular formula of C13H14O6 (calcd. for C14H12O5Na+, 289.0683). The IR spectrum showed absorption bands of C = O stretching at 1713 and 1669 cm−1, and aromatic C = C stretching at 1589, 1477 and 1432 cm−1. The 1H NMR data (Table 1) displayed proton signals of an aromatic at δH 7.67 (1H, s, H-6), an oxygenated methine at δH 4.63 (1H, 4.65 (dqd, J = 12.5, 6.3, 3.2 Hz, H-3), two methylene at δH 3.82 (2H, dd, J = 17.6, 3.2 Hz, H-4) and 3.00 (dd, J = 17.6, 11.7 Hz, H-4), two methoxy at δH 3.94 (3H, s, H-7-OCH3) and 3.89 (3H, s, 5-COOCH3) and a methyl at δH 1.55 (3H, d, J = 6.3 Hz, H-3-CH3). Based on 1H NMR data, the signals of aromatic carbons were in the range of δC 108.9–156.4, oxygenated methine carbon at δC 76.1 (C-3) and methoxy carbons at δC 56.3 (C-7-OCH3) and 52.1 (C-5-COOCH3). In addition, there were carbonyl carbons signals at δC 170.4 (C-1) and 166.2 (C-5-CO). The COSY correlation of H-3 to H-4 and H-3-CH3, and the HMBC correlations of H-3-CH3 to C-3 and C-4, and H-4 to C-3, C-4a and C-8a, suggested a lactone ring. The tetrasubstituted aromatic ring was confirmed by the correlations of H-6 to C-4a, C-5, C-7 and C-8, H-7-OCH3 to C-7 and H-8-OH to C-7, C-8 and C-8a. The above data suggested a mellein analogue of 2. The methoxycarbonyl group at C-5 was confirmed by correlations of H-4 to C-4a and C-5, H-6 to C-5 and C-5-CO and H-5-COOCH3 to C-5-CO, which, together with a hydrogen-bonded hydroxyl proton signal at δH 12.10 (1H, s, H-8-OH), confirmed the presence of a hydroxy group at C-8. The absolute configuration at C-3 was deduced by a comparison of the specific rotation [α]D25.6 -123.6 (c 1.0, CHCl3) of 2 with the known mellein core-structure, which were [α]D24.6 -44.8 (c 1.0, CHCl3) for R and [α]D + 92° (c 1.14, MeOH) for S as observed in a mellein (3) [18]. This was proved through a comparison of experimental and calculated ECD spectra (Fig. 3). The experimental ECD spectrum showed cotton effects at 204 nm (Δε = + 4.52) and 251 nm (Δε =—11.70), which closely matched the calculated 3R isomer. Therefore, it was concluded that 2 was a new mellein derivative, named (3R)-7-methoxy-5-methoxycarbonyl mellein.
Compound 15 was a yellow viscous liquid. The molecular formula of C17H22O5 was deduced from the [M + H]+ peak at m/z 307.1546 in the HRESIMS (calcd. for C17H23O5+, 307.1540). The key IR absorption bands of O–H stretching and C = O stretching of ester were at 3242 cm−1 and 1689 cm−1, respectively. The 1H NMR data (Table 2) showed that there were proton signals of two aromatics at δH 6.40 (1H, s, H-7) and 6.26 (1H, s, H-5), three oxymethines at δH 4.65 (1H,br t, J = 10.5 Hz, H-3), 3.67 (1H, t, J = 10.7 Hz, H-2′) and 3.43 (1H, m, H-6′), a methoxy at δH 3.80 (3H, s, H-8-OCH3), and a methyl at δH 1.29 (3H, d, J = 6.0 Hz, H-6′-CH3).
The spectrum also showed signals of methylene protons at δH 2.76 (1H, dd, J = 16.0, 11.4 Hz, H-4) and 2.67 (1H, d, J = 16.0 Hz, H-4) together with other methylene protons at the range of δH 1.1 to 1.8 (m, H-9 and H-3′ to H-5′). The 13C NMR data (Table 2) showed carbon signals of carbonyl ester at δC 164.4 (C-1), six aromatics at δC 163.5 (C-8), 162.9 (C-6), 144.2 (C-4a), 106.8 (C-5), 105.4 (C-8a) and 98.7 (C-7), and three oxygenated at δC 74.3 (C-3), 74.0 (C-6′) and 73.3 (C-2′).
The COSY correlations (Fig. 2) between H-3 to H-4 and H-9 together with the HMBC correlation of H-4 to C-3, C-4a, C-5, and C-8a, H-5 to C-4, C-7 and C-8a, H-7 to C-5, C-6 and C-8a suggested a 3,4-dihydroisocoumarin structure and a methoxy group at C-8 (δC 163.5). This was confirmed by the correlation of OCH3-8 to C-8. The correlations between H-2′ to H-3′, H-4′ to H-5′ and H-6' to H-6′-CH3 along with the correlation of H-6′-CH3 to C-5′ and C-6′suggested a tetrahydropyran ring. The connections between 3,4-dihydroisocoumarin and tetrahydropyran were confirmed by the correlations between H-9 to H-3 and H-2′. Based on the analysis of the NMR data and the comparison of those to literature, compound 15 resembled the known isocoumarins, (3R,2′R,6′S)-asperentin-8-O-methylether (19) [34]. However, the 13C NMR data of stereogenic centers at C-3 (δC 74.3), C-2′ (δC 73.3) and C-6′ (δC 74.0) shifted to low field, suggested a different stereochemistry. The correlations between H-2′ and H-6′ from NOE experiments, suggested that both were on the same face of the tetrahydropyran ring. The 13C NMR chemical shift calculation of two possible diastereomers were calculated (GIAO calculation using CPAM model in chloroform at the B3LYP/6-31G(d,p) level of theory) and the calculated ECD spectra for 3S,2′R,6′R (15a), 3R,2′S,6′S (15b), 3S,2′S,6′S (15c) and 3R,2′R,6′R (15d) were generated (TD-DFT calculation using CPAM model in DCM at the CAM-B3LYP/6–311 + + G(d,p) level of theory) (See SI). The ECD spectrum generated for a 3S,2′R,6′R (15a) has similarities to the experimental spectrum of 15 (Fig. 3), together with 91.24% of DP4 + (C data) probability analysis, which indicates the absolute configuration of 15 to be 3S,2′R,6′R. It can be concluded that 15 was a new asperentin derivative, named (3S,2′R,6′R)-asperentin-8-O-methylether.
Compound 16 was a brown viscous liquid and the [M + Na]+ peak at m/z 247.1304 in the HRESIMS suggested the molecular formula C13H20O3 (calcd. for C13H20O3Na+, 247.1305). The key IR absorption bands were the C = O stretching of an unsaturated carbonyl ketone at 1666 cm−1 and a C = C stretching at 1610 cm−1. The 1H NMR data (Table 2) showed proton signals of an olefinic at δH 5.32 (1H, s, H-3), three oxymethines at δH 4.57 (1H, m, H-6), 4.07 (1H, m, H-2′) and 3.87 (1H, pd, J = 6.4, 3.2 Hz, H-6′), five methylene at the range of δH 1.33–2.42 (H-5, H-7, H-3′, H-4′ and H-5′), and two methyls at δH 1.99 (3H, s, H-2-CH3) and 1.18 (3H, d, J = 6.4 Hz, CH3-6). The 13C NMR of 16 showed carbon signals of an α,β-unsaturated ketone at δC 193.0, (C-4), two olefinics at δC 174.2 (C-2) and 104.9 (C-3), and three oxymethines at δC 76.2 (C-6), 67.0 (C-6′) and 66.5 (C-2′). The COSY correlations (Fig. 2) between H-5 to H-6 and the HMBC correlation between H-5 to C-4 and C-6, H-3 to C-2, C-5 and C-2-CH3, and H-2-CH3 to C-2 and C-3 indicated a 2,3-dihydropyran-4-one ring. The correlations between H-2′ to H-7, and H-6′ to H-5′ and H-6′-CH3 together with correlations of H-5′ to C-3′ and C-6′, H-6′-CH3 to C-5′ and C-6′ suggested a tetrahydropyran ring. The connection of both rings was confirmed by correlations between H-6, H-7 and H-2′ and between H-5 to C-7 and H-7 to C-2′. The 13C NMR chemical shifts of possible diastereomers were estimated (GIAO calculation using CPAM model in chloroform at the B3LYP/6-31G(d,p) level of theory), and the ECD spectra for 6S,2′R,6′S (16a), 6R,2′S,6′R (16b), 6S,2′S,6′R (16c) and 6R,2′R,6′S (16d) were generated (TD-DFT calculation using CPAM model in DCM at the CAM-B3LYP/6–311 + + G(d,p) level of theory) (See SI). In combination with a DP4 + (C data) probability analysis result of 100% and the ECD spectrum of the 6S,2′R,6′S (16a) isomer, which was similar to the experimental spectrum 16, (Fig. 3), confirmed the absolute configuration of 16 as 6S,2′R,6′S. Thus compound 16 was identified as the new (6S,2′R,6′S)-6-methyl-2-((6-methyltetrahydro-2H-pyran-2-yl)methyl)-2,3-dihydro-4H-pyran-4-one.
Compound 17 was a brown viscous liquid. The molecular formula of C13H18O3 was deduced from the [M + H]+ peak at m/z 223.1320 in the HRESIMS (calcd. for C13H19O3+, 223.1329). The IR spectrum showed absorption bands of O–H stretching at 3269 cm−1 and C = C stretching at 1601 cm−1. From the 1H NMR spectrum, there were signals suggesting the existence of a tetrahydropyran comparable to 16 (Table 2). The additional signals were three aromatic protons at δH 6.23 (2H, d, J = 2.3 Hz, H-4 and H-6) and 6.16 (1H, t, J = 2.3 Hz, H-2), which corresponded to six aromatic carbons signals at δC 156.9 (C-1 and C-3), 108.7 (C-4 and C-6) and 100.9 (C-2). These suggested the presence of a 1,3,5-trisubstituted aromatic ring, which was confirmed by the HMBC correlations (Fig. 2) between H-6 to C-1 and C-7, H-2 to C-1, C-3, C-4 and C-6, and H-4 to C-3. The correlations between H-7 to C-4, C-5, C-6, C-2′ and C-3′ confirmed a connection between tetrahydropyran and the aromatic ring. The 13C NMR chemical shifts of two diastereomers were calculated (GIAO calculation using CPAM model in chloroform at the B3LYP/6-31G(d,p) level of theory) and the results suggested a 2′R,6′S or 2′S,6′R configuration. The calculated ECD spectra of 2′R,6′S (17a), 2′S,6′R (17b), 2′R,6′R (17c) and 2′S,6′S (17d) were generated (TD-DFT calculation using CPAM model in DCM at the CAM-B3LYP/6–311 + + G(d,p) level of theory) (See SI). The ECD spectrum generated for 2′R,6′S (17a) was the best fit to the experimental ECD (Fig. 3), which corresponded to a DP4 + (C data) probability of 100%, confirming the absolute configuration of 17 as 2′R,6′S. These led to the conclusion that compound 17 was a new (2′R,6′S)-5-((-6-methyltetrahydro-2H-pyran-2-yl)methyl)benzene-1,3-diol.
2.2 Antiproliferative activity
The antiproliferative activity against HCT116, HT29, MCF-7, HeLa and Vero cells of compounds 1–5, 15, and 17–22 were evaluated (Table 3), based on the amount isolated, whether they were known or new compounds and if they had similar core structures. Compound 18 showed a strong broad spectrum against all cancer cells and normal cells with the IC50 in the range of 2.09–10.09 µg/mL. Compounds 1, 17 and 20 showed moderate anti-proliferation against all cancer cells but were not toxic toward normal cells, while 5 and 15 were slightly toxic against HCT116, HT29, and HeLa, but not toxic toward MCF-7 and normal cells. Based on the core structure of the tested compounds, for example, asperentin derivatives (15 and 18–22), the phenolic and the hydroxy group on tetrahydropyran may play a role in their antiproliferative activity.
2.3 HPLC analysis of crude extracts from X. cf. longipes SWUF08-81
The compounds isolated from X. cf. longipes SWUF08-81, when cultivated in PBD and YM, were the same. This corresponded to the preliminary examination of crude EtOAc extracts from broth and mycelium by HPLC compound profiling (See SI). The intensity of the cytotoxic compounds 18 and 20 in HPLC profiles were notably different in both media. The production of 18 and 20 were validated by HPLC–DAD, which revealed the considerable difference in their production. The results showed that 18 was produced and seceded into the liquid media, as it was quantified nearly ten times more in broth than mycelium in both media. In addition, it was produced approximately three times more in PDB than the YM broth. Compound 20 was produced only one times more in the YM than the PDB and roughly the same amount was found in both broth and mycelium.
In conclusion, the study of chemical constituents from X. cf. longipes SWUF08-81, cultivated in three media (GM, YM and PDB) revealed fourteen compounds (1–14) from GM, fourteen compounds (15–28) from YM and eight compounds (18, 19–23, 25, 27) from PDB. They were structurally categorized into various groups of various bioactive natural products including dibenzofuran (1), melleins (2–5), cytochalasins (6–10), monocyclic polyketides (11–14), dihydroisocoumarins (15 and 18–22), tetrahydro-2H-pyran derivatives (16 and 17), auroglaucins (23–26), alkaloid (27) and anthraquinone (28), in which five of them (1–2 and 15–17) were undescribed compounds. It is worth noting that the cytochalasins have previously been reported from X. longipes, however there were no diterpenoids found in this work [13,14,15, 35]. The results showed that the production of the compounds in GM medium were entirely different from the YM and PDB media, which may be due to the different sources and carbon and nitrogen ratio [36, 37]. In the YM and PDB, the same group of compounds were identified, however when investigated, the production of 18 and 20 showed that the PDB medium is the best medium to start with to increase the quantity of 18.
This study demonstrated that fungi remain one of the most important sources of a wide variety of bioactive compounds that can be regulated to larger production scales in the laboratory or industry. In addition, the fungus X. cf. longipes SWUF08-81 proved to be a potential source of anticancer agents that could be comprehensively explored in terms of optimizing growth conditions to increase the production of active compounds for molecular level studies.
3 Materials and methods
3.1 General experimental procedures
Melting points were measured on a Gallenkamp SANYO MPU250BM3.5 melting point apparatus (Gallenkamp, London, UK). Optical rotation was recorded on a JASCO DIP-1000 digital polarimeter (JASCO Corporation, Tokyo, Japan). The UV and ECD spectra were measured on a JASCO J-810 CD spectrometer (JASCO Corporation, Tokyo, Japan. IR spectra were obtained using a BRUKER TENSOR 27 FT-IR spectrophotometer (Bruker Optics, Kowloon Bay, Hong Kong). 1H and 13C NMR spectra were recorded on a Varian Mercury plus 400 MHz (Oxford Instruments, Abingdon, UK) and BRUKER 400 and 500 MHz NMR spectrometers (Bruker BioSpin AG, Fällanden, Switzerland). Electrospray Ionization (ESI) mass spectra were measured on a micrOTOF Bruker mass spectrometer (Bruker Daltonics, Kanagawa, Japan). High Performance Liquid Chromatography (HPLC) for separation was performed on a Shodex RI-101 chromatographer equipped with a Hitachi L 6000 pump and Shimadzu SPD-20A UV–Vis detector (Shimadzu, Kyoto, Japan) using a Cosmosil 5SL-II 4.6 ID × 250 mm normal phase column (Nacalai Tesque Inc., Kyoto, Japan. For chemical profiling, HPLC was performed on the Agilent 1220 LC system VL (Agilent, Germany), a binary pump, a manual injector with a sample loop of 10 µL and an Agilent 1260 infinity DAD detector. Column chromatography (CC) and flash column chromatography (FCC) were performed on silica gel 60 (0.063–0.200 mm and less than 0.063 mm mesh, MERCK, Germany). Sephadex LH-20 for chromatography (SIGMA-ALDRICH, Sweden) was used for size exclusion chromatography. Thin layer chromatography (TLC) analysis was carried out on silica gel 60 F254 aluminum sheets. The TLC sheet was visualized under UV light (254 and 366 nm) and it was further stained by anisaldehyde reagent and then heated until spots of compounds appeared (Additional file 1).
3.2 Fungal material
The fungal stroma was collected from Phu Khieo Wildlife Sanctuary in Chaiyaphum Province and deposited at Srinakharinwirot University Fungal Herbarium (SWUF) under the access number of SWUF08-81. The fungal pure culture obtained from ascospores was grown on potato dextrose agar at 30ºC. The morphological characteristics including stromal surface, shape, size and color were carefully observed under stereomicroscope. Its morphology was similar to Xylaria longipes Nitschke described by Rogers except that the ascospore size was slightly smaller (11–14 µM length × 4.5–5 µM width) and the germ slit was straight and ran the full length of the spore [38]. The nucleotide sequences of internal transcribed spacers (ITS) were analyzed and resulted in 100% similarity to X. longipes (AF163038). Therefore, we noted the fungus name as X. cf. longipes, as morphological characteristics of the fungus were slightly different [39]. The ITS sequence was submitted to GenBank database as accession number OP082329.
3.3 Fermentation, extraction, and isolation
The ascospores were isolated from mature fungal stroma and cultivated on potato dextrose agar (PDA). The pure cultures were individually inoculated into 59 L of Glucose-Malt extract (called GM) medium (containing glucose (60 g/L) and malt extract (20 g/L)), 14 L of Yeast extract-Malt extract (called YM) medium (containing yeast extract (3 g/L), malt extract (3 g/L), peptone (5 g/L) and dextrose (10 g/L)) and 14 L of Potato Dextrose Broth (PDB) medium (containing potatoes (200 g/L) and dextrose (20 g/L)). The cultures were kept at room temperature for 45 days and then each of the fermented broths was filtered and partitioned with EtOAc by liquid–liquid extraction. Removal of EtOAc gave crude EtOAc broth extracts from GM medium (128.15 g, 14.48%), YM medium (9.86 g, 18.58%) and PDB medium (5.15 g, 9.90%). The mycelium from the three media were separately dried in an oven at 50 °C for 3 days. The dried mycelium (885.09 g (GM), 53.06 g (YM) and 52.03 g (PDB)) were ground and successively extracted with EtOAc (500 mL × 3) and the removal of solvents gave mycelium crude extracts from GM (45.44 g, 5.13%), YM (7.09 g, 19.37%) and PDB (3.92 g, 7.53%). The purification procedures of the undescribed compounds 1, 2, 15–20 are described herein (for known compounds see SI).
The crude EtOAc extract of GM broth (128.15 g) was purified by silica gel CC eluted with a gradient system of EtOAc-hexane (0:1–1:0) and MeOH-EtOAc (0:1–1:0) to give 10 fractions, GB1-GB10. Fraction GB3 was purified by FCC eluted with a gradient system of EtOAc-hexane (0.5:9.5–1:0) to get 14 fractions, GB3.1-GB3.14. Fraction GB3.7 was separated by FCC eluted with 2:3 EtOAc-hexane to give 2 (109.3 mg, 0.012%). Fractions GB3.12.1-GB3.12.4 were obtained by the fractionation of GB3.12 by FCC eluted with 3:2 EtOAc-CH2Cl2. Fraction GB3.12.1 was purified by PLC developed with 1:7:2 MeOH-CH2Cl2-hexane to yield 1 (13.9 mg, 0.002%).
The crude EtOAc extract from YM broth (9.86 g) was purified by silica gel CC and eluted with a gradient system of EtOAc-hexane (0:1–1:0 v/v) and MeOH-EtOAc (0:1–1:0 v/v) to give 8 fractions, YB1-YB8. Fraction YB2 was purified by FCC eluted with 1:4 EtOAc-hexane to get a further 13 fractions, YB2.1-YB2.13. Three compounds, 17 (18.2 mg, 0.227%), 18 (194.2 mg, 0.366%) and 20 (71.6 mg, 0.135%) were obtained from fractions YB2.13, YB2.10 and YB2.4, respectively. Fraction YB2.9 was further purified by FCC eluted with 0.5:1:8.5 MeOH-EtOAc-hexane to give compound 16 (8.5 mg, 0.016%). Fraction YB4 was purified by CC eluted with a gradient system of EtOAc: hexane (1:4–1:0) to give 8 fractions, YB4.1-YB4.8. Fraction YB4.5 was separated by CC eluted with 2:3 EtOAc-hexane to yield compound 15 (21.3 mg, 0.040%).
The crude EtOAc extract from PDB broth (5.15 g) was purified by silica gel CC eluted with a gradient system of EtOAc-hexane (0:1–1:0 v/v) and MeOH-EtOAc (0:1–1:0 v/v) to give 8 fractions, PB1-PB8. Fraction PB2 was separated by CC eluted with 1:4 EtOAc-hexane to give compound 17 (29.2 mg, 0.015%,), 18 (518.1 mg, 0.269%,) and 20 (7.3 mg, 0.004%,) from fractions PB2.7, PB2.5 and PB2.2, respectively.
3.4 Spectroscopic data of compounds
3.4.1 1,3,8-Trihydroxy-7-methoxy-9-methyldibenzofuran (1)
Brown amorphous solid (MeOH); m.p. 244–245 °C; UV (MeOH) λmax (log ε) 245 (4.23) nm; IR (ATR) νmax 3354, 2929, 2851, 1615, 1462, 1430, 1350, 1152, 1131, 1090, 1064 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z: 283.0597 [M + Na]+ (calcd. for C14H12O5Na+, 283.0577).
3.4.2 (3R)-6-Methoxy-5-methoxycarbonylmellein (2)
White amorphous solid; m.p. 135–136 °C; [α]D25.6 -123.6 (c 1.0, CHCl3); UV (CH2Cl2) λmax (log ε) 238 (4.22) nm; ECD (MeOH) λ(∆ε) 204 (+ 4.52) and 251 (-11.70) nm; IR (ATR) νmax 2960, 2840, 1713, 1669, 1589, 1478, 1433, 1394, 1272, 1226, 1192, 1164 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z: 289.0687 [M + Na]+ (calcd. for C13H14O6Na+, 289.0683).
3.4.3 (3S,2′R,6′R)-Asperentin-8-O-methylether (15)
Yellow viscous liquid; [α]D27.5 + 11.4 (c 1.0, CH2Cl2); UV (MeOH) λmax (log ε) 228 (4.36) nm; ECD (MeOH) λ (∆ε) 225 (+ 5.19), 250 (+ 0.42), 269 (+ 4.10) nm; IR (ATR) νmax 3242, 2931, 2853, 1689, 1609, 1589, 1470, 1440, 1350, 1243, 1168, 1088 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z: 307.1546 [M + H]+ (calcd. for C17H23O5+, 307.1540).
3.4.4 (6S,2′R,6′S)-6-Methyl-2-((6-methyltetrahydro-2H-pyran-2-yl)methyl)-2,3-dihydro-4H-pyran-4-one (16)
Brown viscous liquid; [α]D27.6 -25.6 (c 1.0, CH2Cl2); UV (MeOH) λmax (log ε) 260 (3.94) nm; ECD (MeOH) λ (∆ε) 213 (+ 0.86), 217 (+ 1.05), 259 (-1.46), 289 (-0.36), 314 (-0.89) nm; IR (ATR) νmax 2929, 2867, 1666, 1610, 1462, 1438, 1400, 1337, 1114, 1086, 1034 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z: 247.1304 [M + Na]+ (calcd. for C13H20O3Na+, 247.1305).
3.4.5 (2′R,6′S)-5-((-6-Methyltetrahydro-2H-pyran-2-yl)methyl)benzene-1,3-diol (17)
Brown viscous liquid; [α]D27.8 -38.4 (c 0.1, EtOH); UV (MeOH) λmax (log ε) 226 (3.06) nm; ECD (MeOH) λ (∆ε) 213 (-3.82), 228 (+ 0.76) nm; IR (ATR) νmax 3269, 2971, 2937, 2869, 1601, 1453, 1481, 1339, 1147, 1037, 1004 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z: 223.1320 [M + H]+ (calcd. for C13H19O3+, 223.1329).
3.5 Methylation of compound 1
Compound 1 was methylated using TMS-diazomethane (TCI, Japan) to yield the methylated product 1a. Compound 1 (1.0 mg, 3.84 µM) was dissolved in MeOH. TMS-diazomethane (200 μL) was then added to the solution and kept at room temperature overnight. The complete structure of 1 was deduced by the analysis of NMR spectroscopic data and the NOE experiment of the methylated product (1a).
3.6 Quantitative high-performance liquid chromatography analysis (HPLC analysis)
The chemical profiling by HPLC was performed using Agilent ZORBAX Eclipse C18 (4.6 × 250 mm, 5 µm) column. Data processing was performed using OpenLAB CDS Chemstation software. Gradient elution was achieved with a mobile phase consisting of methanol and DI water, starting at a ratio of 60:40 v/v and increasing up to 100:0 v/v in 50 min at a flow rate of 1.0 mL/min. The injection volume was 10 μL and the mobile phase was filtrated and degassed by sonication before being used. The UV spectra were monitored over a range of 200 to 400 nm, while the chromatograms were recorded at 220 nm to detect all analyzed compounds. All crude extracts and pure compound solutions were prepared by adding HPLC grade methanol and then filtered through a 0.45 µm Nylon membrane. The concentration of crude extract solutions was 5 mg/mL and the chromatograms were identified by comparing their retention times and UV spectra with compounds 18 and 20. Seven different concentrations of 18 and 20 were prepared to generate the calibration curves, which were performed by a two-fold dilution with an initial concentration of 1000 µg/mL to 15.6 µg/mL in triplicates. The validation of analytical methods was determined by linearity range, precision, detection and quantification limits. The linearity was determined by the correlation coefficients of the calibration curves. The limits of detection (LOD) and quantification (LOQ) were calculated based on the standard deviation and slope of the calibration curve. The precision was determined by repeatability (intraday assay) and intermediate precision (inter-day assay). The repeatability was measured by injecting three selected different concentrations at low (125 µg/mL), middle (250 µg/mL) and high (500 µg/mL) concentration of 18 and 20 in triplicate on the same day. Intermediate precision was estimated by analyzing the same solutions in triplicate over a period of three consecutive days. The accuracy of the method was expressed as % recovery. The recoveries of standard solutions at 125, 250 and 500 µg/mL were calculated from the corresponding calibration curve [40].
3.7 Quantum chemical calculations
The quantum chemical calculations including 13C NMR chemical shift and Electronic Circular Dichroism (ECD) calculations were used to determine the absolute configurations. Conformational searches were carried out by Monte Carlo protocol under MM + molecular mechanics force field by HyperChem (HyperChem™ Professional 8.0.4, Hypercube, Inc., Gainesville, FL, USA). Re-optimization of the stable conformers with a Boltzmann distribution over 5% were performed using the density functional theory (DFT) with the B3LYP hybrid functional and 6-31G(d) basis sets [17, 41]. GaussView 6.0 was used to view the optimized structures and modify computation input files for calculation. For 13C NMR calculation, the magnetic shielding tensors of each optimized conformer were calculated using GIAO method at B3LYP functional and 6-31g(d,p) basis set. The solvent effects were analyzed using the Conductor–like Polarisable Continuum Model (CPCM) in chloroform. GaussView 6.0 was used to display the calculated shielding tensors of each nuclei in each conformer. The Boltzmann averaged shielding tensors of each carbon atom were calculated and then input to an Excel file (downloaded from sarotti-nmr.weebly.com) for the DP4 + (C data) probability analysis. For ECD calculation, the transition energies for the valence excited–states and absorption ECD spectra were computed with the time-dependent density functional theory (TD–DFT) method with the CAM–B3LYP long–range corrected functional at the 6–311 + + G(d,p) basis set. The analysis of solvent effects was estimated using C–PCM in DCM. The ECD calculations were performed using the Gaussian 09 program, and calculated ECD spectra were generated using the GaussSum program [42].
3.8 Antiproliferative activity against cancer cell lines
The antiproliferative activity was evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay [43]. Four cancer cell lines; human cervical carcinoma (HeLa), human colon carcinoma (HT29 and HCT116) and human breast carcinoma (MCF-7), along with African green monkey kidney cells (Vero cells) were seeded in 96-well plates (8000 cells/well) and cultured for 24 h, followed by being treated with the selected compounds at 100 µg/mL for 72 h. Following this, 10 μL of MTT reagent (5 mg/mL in phosphate buffer solution (PBS)) was added to each well and further incubated at 37 °C for 2 h. Then, 100 μL of DMSO (dimethyl sulfoxide) was used to dissolve the formazan dye. Finally, the absorbance of the solution was measured using a microplate reader (Bio-Rad Laboratories, Hercules, CA) at wavelengths of 550 and 655 nm. The absorbance at 655 nm was used as a reference wavelength. Compounds that showed strong anti-proliferative activity in the preliminary test, with less than 10% viability, were further determined for the half maximum inhibitory concentration (IC50) values using the same procedures. Cisplatin was used as the positive control drug.
References
Sharma M, Sharma R. Drugs and drug intermediates from fungi: striving for greener processes. Crit Rev Microbiol. 2016;42(2):322–38.
Aly AH, Debbab A, Proksch P. Fifty years of drug discovery from fungi. Fungal Diversity. 2011;50(1):3–19.
Ola AR, Thomy D, Lai D, Brotz-Oesterhelt H, Proksch P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J Nat Prod. 2013;76(11):2094–9.
Zhang H, Deng Z, Guo Z, Tu X, Wang J, Zou K. Pestalafuranones F-J, five new furanone analogues from the endophytic fungus Nigrospora sp. BM-2. Molecules. 2014;19(1):819–25.
Zhang H, Deng Z, Guo Z, Peng Y, Huang N, He H, et al. Effect of culture conditions on metabolite production of Xylaria sp. Molecules. 2015;20(5):7940–50.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803.
Suwannasai N, Sangvichien E, Phosri C, McCloskey S, Wangsawat N, Thamvithayakorn P, et al. Exploring the Xylariaceae and its relatives. Bot Stud. 2023;64(1):15.
Macias-Rubalcava ML, Sanchez-Fernandez RE. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J Microbiol Biotechnol. 2017;33(1):15.
Youssef FS, Simal-Gandara J. Comprehensive overview on the chemistry and biological activities of selected alkaloid producing marine-derived fungi as a valuable reservoir of drug entities. Biomedicines. 2021;9(5):485.
Becker K, Stadler M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot (Tokyo). 2021;74(1):1–23.
Sawadsitang S, Mongkolthanaruk W, Suwannasai N, Sodngam S. Antimalarial and cytotoxic constituents of Xylaria cf. cubensis PK108. Nat Prod Res. 2015;29(21):2033–6.
Sawadsitang S, Suwannasai N, Mongkolthanaruk W, Ahmadi P, McCloskey S. A new amino amidine derivative from the wood-decaying fungus Xylaria cf. cubensis SWUF08–86. Nat Prod Res. 2018;32(19):2260–7.
Wang Q-Y, Chen H-P, Liu J-K. Isopimarane diterpenes from the rice fermentation of the fungicolous fungus Xylaria longipes HFG1018. Phytochem Lett. 2021;45:100–4.
Chen HP, Zhao ZZ, Cheng GG, Zhao K, Han KY, Zhou L, et al. Immunosuppressive nor-isopimarane diterpenes from cultures of the fungicolous fungus Xylaria longipes HFG1018. J Nat Prod. 2020;83(2):401–12.
Chen HP, Li J, Zhao ZZ, Li X, Liu SL, Wang QY, et al. Diterpenes with bicyclo[2.2.2.]octane moieties from the fungicolous fungus Xylaria longipes HFG1018. Org Biomol Chem. 2020;18(13):2410–5.
Fan YZ, Tian C, Tong SY, Liu Q, Xu F, Shi BB, et al. The antifungal properties of terpenoids from the endophytic fungus Bipolaris eleusines. Nat Prod Bioprospect. 2023;13(1):43.
Li J, Liu JK, Wang WX. GIAO 13C NMR calculation with sorted training sets improves accuracy and reliability for structural assignation. J Org Chem. 2020;85(17):11350–8.
Chacón-Morales P, Amaro-Luis JM, Bahsas A. Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus. Av Quim. 2013;8(3):145–51.
Klaiklay S, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Buatong J, Bussaban B. Metabolites from the mangrove-derived fungus Xylaria cubensis PSU-MA34. Arch Pharm Res. 2012;35(7):1127–31.
Deng KZ, Xiong Y, Zhou B, Guan YM, Luo YM. Chemical constituents from the roots of Ranunculus ternatus and their inhibitory effects on Mycobacterium tuberculosis. Molecules. 2013;18(10):11859–65.
Minato H, Katayama T. Studies on the metabolites of Zygosporium masonii. Part II. Structures of zygosporins D, E, F, and G. J Chem Soc Perkin 1. 1970;1:45–7.
Edwards RL, Maitland DJ, Whalley AJS. Metabolites of the higher fungi. Part 24. Cytochalasin N, O, P, Q, and R. New cytochalasins from the fungus Hypoxylon terricola Mill. J Chem Soc, Perkin Trans 1. 1989(1):57–65.
Tansuwan S, Pornpakakul S, Roengsumran S, Petsom A, Muangsin N, Sihanonta P, et al. Antimalarial benzoquinones from an endophytic fungus, Xylaria sp. J Nat Prod. 2007;70(10):1620–3.
Shiono Y, Murayama T, Takahashi K, Okada K, Katohda S, Ikeda M. Three oxygenated cyclohexenone derivatives produced by an endophytic fungus. Biosci Biotechnol Biochem. 2005;69(2):287–92.
Tian JK, Sun F, Cheng YY. Chemical constituents from the roots of Ranunculus ternatus. J Asian Nat Prod Res. 2006;8(1–2):35–9.
Kimura Y, Shimomura N, Tanigawa F, Fujioka S, Shimada A. Plant growth activities of aspyran, asperentin, and its analogues produced by the fungus Aspergillus sp. Z Naturforsch, C. , J Biosci. 2012;67(11–12):587–93.
Zheng H, Zhao C, Fang B, Jing P, Yang J, Xie X, et al. Asymmetric total synthesis of cladosporin and isocladosporin. J Org Chem. 2012;77(13):5656–63.
Fujimoto H, Sumino M, Okuyama E, Ishibashi M. Immunomodulatory constituents from an Ascomycete Chaetomium seminudum. J Nat Prod. 2004;67(1):98–102.
Miyake Y, Ito C, Itoigawa M, Osawa T. Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi). Biosci Biotechnol Biochem. 2009;73(6):1323–7.
Li D-L, Li X-M, Li T-G, Dang H-Y, Wang B-G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv Chim Acta. 2008;91(10):1888–93.
Danielsen K, Aksnes DW, Francis GW. NMR study of some anthraquinones from rhubarb. Magn Reson Chem. 1992;30(4):359–60.
Kim JM, Ko RK, Hyun JW, Lee NH. Identification of new dibenzofurans from Distylium racemosum. Bull Korean Chem Soc. 2009;30(1):261–3.
Ko RK, Kim GO, Hyun CG, Jung DS, Lee NH. Compounds with tyrosinase inhibition, elastase inhibition and DPPH radical scavenging activities from the branches of Distylium racemosum Sieb. et Zucc. Phytother Res. 2011;25(10):1451–6.
Wang X, Radwan MM, Tarawneh AH, Gao J, Wedge DE, Rosa LH, et al. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J Agric Food Chem. 2013;61(19):4551–5.
Li J, Wang WX, Chen HP, Li ZH, He J, Zheng YS, et al. (+/-)-Xylaridines A and B, highly conjugated alkaloids from the fungus Xylaria longipes. Org Lett. 2019;21(5):1511–4.
Casas López JL, Sánchez Pérez JA, Fernández Sevilla JM, Acién Fernández FG, Molina Grima E, Chisti Y. Production of lovastatin by Aspergillus terreus: effects of the C: N ratio and the principal nutrients on growth and metabolite production. Enzyme Microb Technol. 2003;33(2–3):270–7.
Shariati S, Zare D, Mirdamadi S. Screening of carbon and nitrogen sources using mixture analysis designs for carotenoid production by Blakeslea trispora. Food Sci Biotechnol. 2019;28(2):469–79.
Rogers JD. Xylaria bulbosa, Xylaria curta, and Xylaria longipes in continental United States. Mycologia. 1983;75(3):3792687.
White T, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications; 1990. p. 315–22.
Uchoa PKS, de Lima LB, Pimenta ATA, de Oliveira MDCF, Mafezoli J, Lima MAS. HPLC quantification of cytotoxic compounds from Aspergillus niger. J Chem. 2017;2017:1–7.
Becke AD. Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98(7):5648–52.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 09, revision B.01. Wallingford: Gaussian Inc.; 2010.
Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell viability assays. Cell viability assays. Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2016.
Acknowledgements
This research was supported by the Fundamental Fund of Khon Kaen University. We would like to gratefully acknowledge the Development and Promotion of Science and Technology Talents Project (DPST) and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, for their financial support.
Author information
Authors and Affiliations
Contributions
Kittiwan Sresuksai, Sasiphimol Sawadsitang, Pakin Noppawan, and Audomsak Churat carried out the experiments, including isolation, purification and structure elucidation. Nuttika Suwannasai, Wiyada Mongkolthanaruk and Kittiwan Sresuksai collected, isolated, identified and cultivated the fungi sample. Thanaset Senawong, Pakin Noppawan and Sasiphimol Sawadsitang carried out the biological activity assay. Audomsak Churat , Sarawut Tontapha, Pairot Moontragoon, and Vittaya Amornkitbamrung computed and analyzed the quantum chemical calculation data. Sirirath McCloskey, Phongphan Jantaharn and Kittiwan Sresuksai planned the scope of the experiment, analyzed all results, prepared and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Separation of known compounds (3–14 and 18–28). Spectroscopic data of all isolated compounds. The 13C NMR chemical shift calculations and ECD spectra of 15–17. HPLC identification of asperentin (18) and (3R,2'R,6'S)-asperentin-6-O-methylether (20) and method validation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sresuksai, K., Sawadsitang, S., Jantaharn, P. et al. Antiproliferative polyketides from fungus Xylaria cf. Longipes SWUF08-81 in different culture media. Nat. Prod. Bioprospect. 14, 6 (2024). https://doi.org/10.1007/s13659-023-00427-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-023-00427-7